Abstract
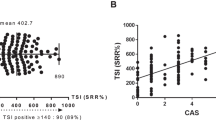
The Thyrotropin receptor antibody (TRAb) is the main driver of Graves’ disease (GD) and its most common extra-thyroidal manifestation: thyroid eye disease (TED). Though key to diagnosis, it has not been used routinely as a marker of disease activity or to guide treatment. Here we demonstrate, through a retrospective review of 105 patients with TED, that serial TRAb levels vary with time, correlate with disease activity and are affected by smoking and endocrine control. Such serial measurements can guide the modern management of thyroid eye disease, helping to prevent the more serious manifestations. We show that surgical thyroidectomy is associated with a reduction in antibody levels and a reduced rate of TED reactivation when compared to radio-iodine ablation where the stimulating antigen is not removed. This provides a molecular explanation for epidemiological studies showing radio-ablation being associated with an increased risk of orbitopathy. To demonstrate the effect of our clinical approach on a patient population, we then compared the incidence and severity of TED in a clinic in a period before and after the introduction of serial TRAb measurements. Despite an increase in disease incidence and severity at presentation over the two-decade study period, our approach saw a significant reduction in the need for surgical intervention for this orbital disorder.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Farid M, Roch-Levecq AC, Levi L, Brody BL, Granet DB, Kikkawa DO. Psychological disturbance in graves ophthalmopathy. Arch Ophthalmol. 2005;123:491–6.
Abraham-Nordling M, Byström K, Törring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165:899–905.
Laurberg P, Berman DC, Bülow Pedersen I, Andersen S, Carlé A. Incidence and clinical presentation of moderate to severe graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J Clin Endocrinol Metab. 2012;97:2325–32.
Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5:177–94.
Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol. 2001;55:283–303.
Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–44.
Dolman PJ. Grading severity and activity in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34(4S Suppl 1):S34–S40.
Utech CI, Khatibnia U, Winter PF, Wulle KG. MR T2 relaxation time for the assessment of retrobulbar inflammation in Graves’ ophthalmopathy. Thyroid. 1995;5:185–93.
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61.
Adams DD, Purves HD, Sirett NE, Beaven DW. The presence of a short-acting abnormal thyroid stimulator in the blood of a thyrotoxic patient. J Clin Endocrinol Metab. 1962;22:623–6.
Konuk O, & Anagnostis. Diagnosis and differential diagnosis in Graves’ Orbitopathy. In: Wiersinga M, Kahaly GJ, editors. Graves’ orbitopathy: a multidisciplinary approach – questions and answers. Basel: Karger; 2017. p. 83.
Salvi M, Berchner-Pfannschmidt U, Ludgate M. Pathogenesis. In: Wiersinga WM, Kahaly GJ, editors. Graves’ orbitopathy: a multidisciplinary approach – questions and answers. Basel: Karger; 2017. p.50.
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ. Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology. 2011;118:2279–85.
Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–70.
Lantz M, Planck T, Asman P, Hallengren B. Increased TRAb and/or low anti-TPO titers at diagnosis of graves’ disease are associated with an increased risk of developing ophthalmopathy after onset. Exp Clin Endocrinol Diabetes. 2014;122:113–7.
Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol. 2000;52:267–71.
Khoo DH, Ho SC, Seah LL, Fong KS, Tai ES, Chee SP, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–80.
Mukasa K, Yoshimura Noh J, Kouzaki A, Ohye H, Kunii Y, Watanabe N, et al. TSH receptor antibody titers measured with a third-generation assay did not reflect the activity of Graves’ ophthalmopathy in untreated Japanese Graves’ disease patients. Endocr J. 2016;63:151–7.
Goldberg R. 48th Cambridge Ophthalmological Symposium: Thyroid and the Eye. 6–7th September 2018, Cambridge UK.
Vestergaard P. Smoking and thyroid disorders—a meta-analysis. Eur J Endocrinol. 2002;146:153–61.
Wiersinga WM. Smoking and thyroid. Clin Endocrinol. 2013;79:145–51.
Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21:168–99.
Bartalena L, Marcocci C, Tanda ML, Manetti L, Dell’Unto E, Bartolomei MP, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998;129:632–5.
Eckstein A, Quadbeck B, Mueller G, Rettenmeier AW, Hoermann R, Mann K, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–6.
Burlacu M, Daumerie C. Epidemiology. In: Wiersinga M, Kahaly GJ, editors. Graves’ orbitopathy: a multidisciplinary approach – questions and answers. Basel: Karger; 2017. p. 35
Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, et al. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves’ disease. JAMA Ophthalmol. 2015;133:290–6.
Meyer Zu Horste M, Pateronis K, Walz MK, Alesina P, Mann K, Schott M, et al. The effect of early thyroidectomy on the course of active Graves’ orbitopathy (GO): a retrospective case study. Horm Metab Res. 2016;48:433–9.
Kahaly G, Schrezenmeir J, Krause U, Schweikert B, Meuer S, Muller W, et al. Ciclosporin and prednisone v. prednisone in treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Invest. 1986;16:415–22.
Weetman AP, McGregor AM, Ludgate M, Beck L, Mills PV, Lazarus JH, et al. Cyclosporin improves Graves’ ophthalmopathy. Lancet. 1983;2:486–9.
Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, Koornneef L, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–9.
Meyer PA. Avoiding surgery for thyroid eye disease. Eye. 2006;20:1171–7.
Leövey A, Bakó G, Szabó J, Kálmán K, Fórizs E. Combined cyclosporin-A and methylprednisolone treatment of Graves’ ophthalmopathy. Acta Med Hung. 1992;49(3–4):179–85.
Weissel M, Zielinski CC, Hauff W, Till P. Combined therapy with cyclosporin A and cortisone in endocrine Basedow endocrine orbitopathy: successful use in compressive optic neuropathy. Acta Med Austria. 1993;20:9–13.
Utech C, Wulle KG, Panitz N, Kiefer H. Immunosuppressive treatment of Graves’ ophthalmopathy with cyclosporine A. Transplant Proc. 1988;20(3 Suppl 4):173–7.
Crisp M, Starkey KJ, Lane C, Ham J, Ludgate M. Adipogenesis in thyroid eye disease. IOVS. 2000;41:3249–55.
Diana T, Wüster C, Kanitz M, Kahaly GJ. Highly variable sensitivity of five binding and two bio-assays for TSH-receptor antibodies. J Endocrinol Invest. 2016;39:1159–65.
Kahaly GJ, Diana T, Glang J, Kanitz M, Pitz S, König J. Thyroid stimulating antibodies are highly prevalent in Hashimoto’s thyroiditis and associated orbitopathy. J Clin Endocrinol Metab. 2016;101:1998–2004.
Acknowledgements
We would like to thank Dr Clive Edelsten for valuable advice and support.
Authors contributions
Dr Murthy conceived of the idea for this study. All authors have contributed to the data gathering, analysis and writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are oculoplastic surgeons who manage patients with Thyroid Eye disease.
Guarantor
Dr Murthy serves as guarantor of this work. It is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted.
Rights and permissions
About this article
Cite this article
Roos, J.C.P., Paulpandian, V. & Murthy, R. Serial TSH-receptor antibody levels to guide the management of thyroid eye disease: the impact of smoking, immunosuppression, radio-iodine, and thyroidectomy. Eye 33, 212–217 (2019). https://doi.org/10.1038/s41433-018-0242-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0242-9
This article is cited by
-
Rapid multiparametric quantitative MRI for predicting the activity of thyroid-associated ophthalmopathy: combination with clinical characteristics
European Radiology (2025)
-
Role of diffusion tensor imaging of extra ocular muscles and orbital fat in Graves’s ophthalmopathy and relation to disease activity
Egyptian Journal of Radiology and Nuclear Medicine (2024)
-
An Appraisal of the Preventive Effect of Statins on the Development of Graves’ Ophthalmopathy: A Hospital-Based Cohort Study
Ophthalmology and Therapy (2024)
-
Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience
Pharmacological Reports (2024)
-
Clinical relevance of thyroid-stimulating immunoglobulin as a biomarker of the activity of thyroid eye disease
Eye (2023)