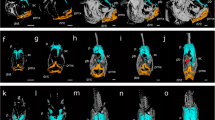
Abstract
While discussion of interactions between the thyroid gland and the eye mostly focus on thyroid eye disease, the involvement of the thyroid and its hormones on development of the eye before birth is also an important perspective that should not be forgotten. Experimental models involving amphibians and fish are valuable both because the developing larvae are not constrained within the adult but can be observed and manipulated as they grow autonomously and also because they metamorphose with substantial morphological changes driven by the hypothalamo-pituitary-thyroid axis occurring as they develop from eggs to adults. In this paper we will first discuss changes seen in the axolotl, a naturally occurring neotonous hypothyroid salamander and the Xenopus toad a widely used laboratory amphibian. Secondly we will document ocular changes in flatfish which exhibit remarkable anatomical alterations during their change from a pelagic to benthic lifestyle. Finally we will evaluate ocular changes in developing zebrafish when subjected to thyroid-disrupting chemicals. These experiments show the influence of the thyroid on ocular development when thyroid function is altered which may be of value in determining changes in human hypothyroid infants but are also important to note as these chemicals are widely used in plastics and also as flame retardants used to control wildfires. Run-off into water courses can damage both wildlife and humans consuming contaminated water and thyroid disruption may have significant effects on reproduction and development, although influences on ocular morphology have yet to be investigated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wulf A The invention of nature: Alexander von Humboldt’s New World. Knopf; 2015.
Reiß C, Olsson L, Hoßfeld UW. The history of the oldest self‐sustaining laboratory animal: 150 years of axolotl research. J Exp Zool Part B: Mol Dev Evol. 2015;324:393–404.
Dunbar GS. “The compass follows the flag”: the French scientific mission to Mexico, 1864–1867. Ann Assoc Am Geogr. 1988;78:229–40.
Neumann FJ. The dragon and the dog: two symbols of time in Nahuatl religion. Numen. 1975;22:1.
Smith HM. The Mexican axolotl: some misconceptions and problems. Bioscience. 1969;19:593–615.
Dumeril A. Observations sur la reproductions dans la menagerie des reptiles du Museum d’Histoire Naturelle, des axolotls, batraciens urodeles a branchies exterieures du Mexique, sur leur developement et sur leur metamorphoses. Nouv Arch Mus Hist Nat. 1866;2:265–92.
Duméril MA. Experiments on the axolotl. Ann Mag Nat Hist. 1867;20:446–9.
Gudernatsch JF. Feeding experiments on tadpoles. Arch für Entwickl der Org. 1912;35:457–83.
Huxley JS. Metamorphosis of axolotl caused by thyroid-feeding. Nature. 1920;104:435.
Babak E. Einige gedanken uber die Beziehung der metamorphose bei den Amphibien zur inneren secretion. Zent fur Physiol. 1913;27:536–41.
Mørch JR. Standardization of thyroid preparations. J Physiol. 1929;67:221–41.
Varga M. The Doctor of Delayed Publications: The Remarkable Life of George Streisinger (1927–1984). Zebrafish. 2018;15:314–9.
Grunwald DJ, Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717.
Fadool JM, Brockerhoff SE, Hyatt GA, Dowling JE. Mutations affecting eye morphology in the developing zebrafish (Danio rerio). Dev Genet. 1997;20:288–95.
De Groef B, Grommen SV, Darras VM. Forever young: endocrinology of paedomorphosis in the Mexican axolotl (Ambystoma mexicanum). Gen Comp Endocrinol. 2018;266:194–201.
Nowoshilow S, Schloissnig S, Fei JF, Dahl A, Pang AW, Pippel M, et al. The axolotl genome and the evolution of key tissue formation regulators. Nature. 2018;554:50–7.
Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19:83.
RioTsonis KD, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–24.
Crawford K, Vincenti DM. Retinoic acid and thyroid hormone may function through similar and competitive pathways in regenerating axolotls. J Exp Zool. 1998;282:724–38.
Thompson CK, Cline HT. Thyroid hormone acts locally to increase neurogenesis, neuronal differentiation, and dendritic arbor elaboration in the tadpole visual system. J Neurosci. 2016;36:10356–75.
Havis E, Le Mevel S, Dubois GM, Shi DL, Scanlan TS, Demeneix BA, et al. Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J. 2006;25:4943–51.
Mai W, Janier MF, Allioli N, Quignodon L, Chuzel T, Flamant F, et al. Thyroid hormone receptor α is a molecular switch of cardiac function between fetal and postnatal life. Proc Natl Acad Sci. 2004;101:10332–7.
Schreiber AM. Flatfish: an asymmetric perspective on metamorphosis. Curr Top Dev Biol. 2013;103:167–94.
Power DM, Einarsdóttir IE, Pittman K, Sweeney GE, Hildahl J, Campinho MA, et al. The molecular and endocrine basis of flatfish metamorphosis. Rev Fish Sci. 2008;16(sup1):95–111.
Isorna E, Obregon MJ, Calvo RM, Vázquez R, Pendón C, Falcón J, et al. Iodothyronine deiodinases and thyroid hormone receptors regulation during flatfish (Solea senegalensis) metamorphosis. J Exp Zool Part B: Mol Dev Evol. 2009;312:231–46.
Okada N, Takagi Y, Tanaka M, Tagawa M. Fine structure of soft and hard tissues involved in eye migration in metamorphosing Japanese flounder (Paralichthys olivaceus). Anat Rec Part A. 2003;273:663–8.
Schreiber AM. Asymmetric craniofacial remodelling and lateralized behavior in larval flatfish. J Exp Biol. 2006;209:610–21.
Alt B, Reibe S, Feitosa NM, Elsalini OA, Wendl T, Rohr KB. Analysis of origin and growth of the thyroid gland in zebrafish. Dev Dyn. 2006;235:1872–83.
Opitz R, Maquet E, Zoenen M, Dadhich R, Costagliola S. TSH receptor function is required for normal thyroid differentiation in zebrafish. Mol Endocrinol. 2011;25:1579–99.
Reider M, Connaughton VP. Effects of low‐dose embryonic thyroid disruption and rearing temperature on the development of the eye and retina in zebrafish. Birth Defects Res Part B: Dev Reprod Toxicol. 2014;101:347–54.
Flint S, Markle T, Thompson S, Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manag. 2012;104:19–34.
Richardson SD, Kimura SY. Emerging environmental contaminants: challenges facing our next generation and potential engineering solutions. Environ Technol Innov. 2017;8:40–56.
Liu W, Zhang X, Wei P, Tian H, Wang W, Ru S. Long‐term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio). J Appl Toxicol. 2018;38:248–58.
Baumann L, Ros A, Rehberger K, Neuhauss SC, Segner H. Thyroid disruption in zebrafish (Danio rerio) larvae: Different molecular response patterns lead to impaired eye development and visual functions. Aquat Toxicol. 2016;172:44–55.
Haq I, Raj A. Endocrine-Disrupting Pollutants in Industrial Wastewater and Their Degradation and Detoxification Approaches. Emerging and Eco-Friendly Approaches for Waste Management. Singapore: Springer; 2019. pp. 121–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Williams, D.L. From axolotl to zebrafish: a comparative approach to the study of thyroid involvement in ocular development. Eye 33, 218–222 (2019). https://doi.org/10.1038/s41433-018-0288-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0288-8