Abstract
Background/Objectives
Choroidal thinning has been suggested in Leber’s hereditary optic neuropathy (LHON). No study has been conducted of the choroid in relation to the retinal ganglion cell-inner plexiform layer (RGC-IPL). We sought to measure choroidal thickness in chronic LHON and to correlate thickness changes with the RGC-IPL.
Subjects/Methods
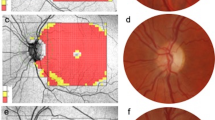
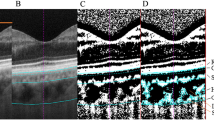
Chronic LHON, 11778 mitochondrial DNA (mtDNA) mutation, patients (26 eyes; mean age: 35.1 ± 16.1 years) were prospectively recruited at Doheny Eye Center, University of California Los Angeles from March 2016 to July 2017. Age-matched healthy controls (27 eyes; mean age: 32.4 ± 11.1 years) were enroled for comparison. Swept-source optical coherence tomography (SS-OCT) imaging was performed in chronic LHON patients and compared with age-matched healthy controls.
Results
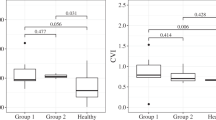
The macular choroid was significantly thinner in chronic LHON (250.5 ± 62.2 μm) compared with controls (313.9 ± 60.2 μm; p < 0.0001). The peripapillary choroid was also significantly thinner in chronic LHON (135.7 ± 51.4 μm) compared with controls (183.0 ± 61.8 μm, p < 0.001). Choroidal thickness strongly correlated with retinal nerve fibre layer (RNFL) thickness in both the macular (R2 = 0.72; 95% CI, 0.57–0.84) and peripapillary regions (R2 = 0.53; 95% CI, 0.31–0.70). Choroidal thickness was also significantly correlated with macular RGC-IPL thickness (R2 = 0.51; 95% CI, 0.26–0.73).
Conclusions
Choroidal thinning in chronic LHON correlated strongly with both RNFL and RGC-IPL thicknesses. These findings may suggest a pathophysiological mechanism involving vascular pathology of the choroid in relation to the retinal ganglion cell complex in LHON.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Leber T. About hereditary and congenital optic nerve disorders. Albrect Von Graefes Arch Clin Exp Ophthalmol. 1871;17:249–91.
Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies – disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114.
Newman NJ. Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol. 2005;140:517–23.
Mackey DA, Oostra RJ, Rosenberg T, Nikoskelainen E, Bronte-Stewart J. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59:481–5.
Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, et al. Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–7.
Barboni P, Savini G, Valentino ML, Montagna P, Cortelli P, De Negri AM, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leberʼs hereditary optic neuropathy. Ophthalmology. 2005;112:120–6.
Nikoskelainen EK, Huoponen K, Juvonen V, Lamminen T, Nummelin K, Savontaus ML. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmology. 1996;103:504–14.
Nikoskelainen E, Hoyt WF, Nummelin K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy. I. Fundus findings in asymptomatic family members. Arch Ophthalmol. 1982;100:1597–602.
Nikoskelainen E, Hoyt WF, Nummelin K. Ophthalmoscopic findings in Leber’s hereditary optic neuropathy: II. The fundus findings in the affected family members. Arch Ophthalmol. 1983;101:1059–68.
Nikoskelainen E, Nummelin K, Hoyt WF, Schatz H. Fundus findings in Leber’s hereditary optic neuroretinopathy III. Fluorescein angiographic studies. Arch Ophthalmol. 1984;102:981–9.
Borrelli E, Triolo G, Cascavilla ML, La Morgia C, Rizzo G, Savini G, et al. Changes in choroidal thickness follow the RNFL changes in Leber's hereditary optic neuropathy. Sci Rep. 2016;6:37332.
Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89.
Sadun AA. Mitochondrial optic neuropathies. J Neurol Neurosurg Psychiatry. 2002;72:423–5.
Carelli V, La Morgia C, Iommarini L, Carroccia R, Mattiazzi M, Sangiorg S, et al. Mitochondrial optic neuropathies: how two genomes may kill the same cell type? Biosci Rep. 2007;27:173–84.
Chevrollier A, Guillet V, Loiseau D, Gueguen N, de Crescenzo MA, Verny C, et al. Hereditary optic neuropathies share a common mitochondrial coupling defect. Ann Neurol. 2008;63:794–8.
Dastiridou AI, Bousquet E, Kuehlewein L, Tepelus T, Monnet D, Salah S, et al. Choroidal imaging with swept-source optical coherence tomography in patients with birdshot chorioretinopathy: choroidal reflectivity and thickness. Ophthalmology. 2017;124:1186–95.
Carelli V, La morgiaC, Valentino ML, Barboni P, Ross-cisneros FN, Sadun AA. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta. 2009;1787:518–28.
Sadun AA, Carelli V, La Morgia C, Karanjia R. Leber's hereditary optic neuropathy (LHON) mtDNA mutations cause cell death by overproduction of reactive oxygen species. Acta Ophthalmol. 2015;93:S255.
Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–61.
Balducci N, Savini G, Cascavilla ML, La Morgia C, Triolo G, Giglio R, et al. Macular nerve fibre and ganglion cell layer changes in acute Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2016;100:1232–7.
Jurkute N, Yu-wai-man P. Leber hereditary optic neuropathy: bridging the translational gap. Curr Opin Ophthalmol. 2017;28:403–9.
Borrelli E, Balasubramanian S, Triolo G, Barboni P, Sadda SR, Sadun AA. Topographic macular microvascular changes and correlation with visual loss in chronic leber hereditary optic neuropathy. Am J Ophthalmol. 2018;192:217–28.
Wong-Riley M. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116.
Yu DY, Yu PK, Cringle SJ, Kang MH, Su EN. Functional and morphological characteristics of the retinal and choroidal vasculature. Prog Retin Eye Res. 2014;40:53–93.
Yu DY, Cringle SJ, Yu PK, Balaratnasingam C, Mehnert A, Sarunic M, et al. Retinal capillary perfusion: spatial and temporal heterogeneity. Prog Retin Eye Res. 2019;70:23–54.
Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA. 1980;77:6715–9.
Wallace D, Singh G, Lott M, Hodge JA, Schurr TG, Lezza AM, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–30.
Sadun AA, La Morgia C, Carelli V. Mitochondrial optic neuropathies: our travels from bench to bedside and back again. Clin Exp Ophthalmol. 2013;41:702–12.
Pan BX, Ross-Cisneros FN, Carelli V, Rue KS, Salomao SR, Moraes-Filho MN, et al. Mathematically modeling the involvement of axons in Leber's hereditary optic neuropathy. Investig Ophthalmol Vis Sci. 2012;53:7608–17.
Borrelli E, Lonngi M, Balasubramanian S, Tepelus TC, Baghdasaryan E, Iafe NA, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2018;39:1216–24.
Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61.
Acknowledgements
We would like to thank the Doheny Eye Center and UCLA for providing a collaborative effort in understanding a unique cohort of participants affected with LHON.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Darvizeh, F., Asanad, S., Falavarjani, K.G. et al. Choroidal thickness and the retinal ganglion cell complex in chronic Leberʼs hereditary optic neuropathy: a prospective study using swept-source optical coherence tomography. Eye 34, 1624–1630 (2020). https://doi.org/10.1038/s41433-019-0695-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-019-0695-5