Abstract
Purpose
The purpose of this retrospective case-control study was to evaluate the relationship between foveal structure, function, microvascular morphology and visual acuity in school-age children with laser-treated retinopathy of prematurity (ROP).
Methods
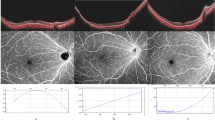
Foveal structural parameters, including the central foveal thickness (CFT), inner retinal thickness (IRT) and outer retinal thickness (ORT), were measured on B-scans using an Optovue XR Avanti optical coherence tomography device. Foveal microvascular parameters, including the foveal avascular zone (FAZ), superficial capillary plexus-vessel density (SCP-VD) and deep capillary plexus-vessel density (DCP-VD), were measured on optical coherence tomography angiography with a scan size of 3 × 3. The P1 amplitudes and P1 implicit times were recorded by a multifocal electroretinogram with 61 elements.
Results
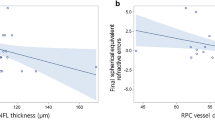
Fifty-five eyes (26 eyes of school-age ROP children and 29 eyes of full-term controls) were analysed. The ROP children manifested a significantly smaller FAZ, higher SCP-VD and higher DCP-VD than the controls (p < 0.001). The CFT (p < 0.001), IRT (p < 0.001) and ORT (p = 0.001) were significantly increased in the ROP group. The P1 amplitudes in all five-ring retinal regions were significantly smaller in the ROP group (p < 0.001). Multivariable analysis indicated that best-corrected visual acuity was positively correlated with post-menstrual age (PMA) and negatively correlated with SCP-VD and CFT (R2 = 0.529, p < 0.001, 0.043 and 0.020, respectively).
Conclusion
The foveal structure, function and microvascular morphology are affected in school-age children with laser-treated ROP. PMA, foveal structural anomalies and microvascular changes in ROP children were associated with impaired visual function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barnaby AM, Hansen RM, Moskowitz A, Fulton AB. Development of scotopic visual thresholds in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4854–60.
Fiess A, Janz J, Schuster AK, Kolb-Keerl R, Knuf M, Kirchhof B, et al. Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefes Arch Clin Exp Ophthalmol. 2017;255:1433–42.
Dubis AM, Costakos DM, Subramaniam CD, Godara P, Wirostko WJ, Carroll J, et al. Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol. 2012;130:1291–300.
Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–12.
Maldonado RS, O’Connell RV, Sarin N, Freedman SF, Wallace DK, Cotten CM, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118:2315–25.
Stoica F, Chirita-Emandi A, Andreescu N, Stanciu A, Zimbru CG, Puiu M. Clinical relevance of retinal structure in children with laser-treated retinopathy of prematurity versus controls—using optical coherence tomography. Acta Ophthalmol 2018;96:e222–8.
Ecsedy M, Szamosi A, Karko C, Zubovics L, Varsanyi B, Nemeth J, et al. A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci. 2007;48:5207–11.
Chen YH, Lien R, Chiang MF, Huang CY, Chang CJ, Wang NK, et al. Outer retinal structural alternation and segmentation errors in optical coherence tomography imaging in patients with a history of retinopathy of prematurity. Am J Ophthalmol. 2016;166:169–80.
Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–56.
Balasubramanian S, Borrelli E, Lonngi M, Velez F, Sarraf D, Sadda SR, et al. Visual function and optical coherence tomography angiography features in children born preterm. Retina. 2018;39:2233–39.
Nonobe N, Kaneko H, Ito Y, Takayama K, Kataoka K, Tsunekawa T, et al. Optical coherence tomography angiography of the foveal avascular zone in children with a history of treatment-requiring retinopathy of prematurity. Retina. 2019;39:111–7.
Falavarjani KG, Iafe NA, Velez FG, Schwartz SD, Sadda SR, Sarraf D, et al. Optical coherence tomography angiography of the fovea in children born preterm. Retina. 2017;37:2289–94.
Chen YC, Chen YT, Chen SN. Foveal microvascular anomalies on optical coherence tomography angiography and the correlation with foveal thickness and visual acuity in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2019;257:23–30.
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS. et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Opthalmol. 2012;124:1–13.
Michalczuk M, Urban B, Chrzanowska-Grenda B, Ozieblo-Kupczyk M, Bakunowicz-Lazarczyk A, Kretowska M. The assessment of multifocal ERG responses in school-age children with history of prematurity. Doc Ophthalmol. 2016;132:47–55.
Akerblom H, Andreasson S, Holmstrom G. Macular function in preterm children at school age. Doc Ophthalmol. 2016;133:151–7.
Fulton AB, Hansen RM, Moskowitz A, Barnaby AM. Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol. 2005;111:7–13.
Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–85.
Altschwager P, Moskowitz A, Fulton AB, Hansen RM. Multifocal ERG responses in subjects with a history of preterm birth. Invest Ophthalmol Vis Sci. 2017;58:2603–8.
Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005;22:171–85.
Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004;21:53–62.
Vajzovic L, Hendrickson AE, O’Connell RV, Clark LA, Tran-Viet D, Possin D, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154:779–89.e2.
Gariano RF. Special features of human retinal angiogenesis. Eye. 2010;24:401–7.
Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821.
Moskowitz A, Hansen RM, Fulton AB. Retinal, visual, and refractive development in retinopathy of prematurity. Eye Brain. 2016;8:103–11.
Engerman RL. Development of the macular circulation. Invest Ophthalmol. 1976;15:835–40.
Springer AD, Hendrickson AE. Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retinal and pars plana growth. Vis Neurosci. 2004;21:775–90.
Kozulin P, Natoli R, Bumsted O’Brien KM, Madigan MC, Provis JM. The cellular expression of antiangiogenic factors in fetal primate macula. Invest Ophthalmol Vis Sci. 2010;51:4298–306.
Bohm MR, Hodes F, Brockhaus K, Hummel S, Schlatt S, Melkonyan H, et al. Is Angiostatin Involved in Physiological Foveal Avascularity? Invest Ophthalmol Vis Sci. 2016;57:4536–52.
Henkind P, Bellhorn RW, Murphy ME, Roa N. Development of macular vessels in monkey and cat. Br J Ophthalmol. 1975;59:703–9.
Hendrickson A, Kupfer C. The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol Vis Sci. 1976;15:746–56.
Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54:549–80.
Villegas VM, Capo H, Cavuoto K, McKeown CA, Berrocal AM. Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol. 2014;158:508–12.e2.
Balasubramanian S, Beckmann J, Mehta H, Sadda SR, Chanwimol K, Nassisi M, et al. Relationship between retinal thickness profiles and visual outcomes in young adults born extremely preterm: The EPICure@19 Study. Ophthalmology. 2019;126:107–12.
Shao Z, Dorfman AL, Seshadri S, Djavari M, Kermorvant-Duchemin E, Sennlaub F, et al. Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011;52:6238–48.
Balasubramanian S, Beckmann J, Mehta H, Sadda SR, Chanwimol K, Nassisi M, et al. Relationship between retinal thickness profiles and visual outcomes in young adults born extremely preterm: The EPICure@19 Study. Ophthalmology. 2018;126:107–12.
Gursoy H, Bilgec MD, Erol N, Basmak H, Colak E. The macular findings on spectral-domain optical coherence tomography in premature infants with or without retinopathy of prematurity. Int Ophthalmol. 2016;36:591–600.
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liang, Z., Yao, Y., Sun, G. et al. Foveal structure, function and microvascular morphology in school-age children with laser-treated retinopathy of prematurity. Eye 35, 1605–1613 (2021). https://doi.org/10.1038/s41433-020-01127-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01127-z