Abstract
Objectives
To compare the effects of intensive and standard statin therapy on severity of diabetic retinopathy (DR) complicated by hypercholesterolaemia in a prespecified substudy of the standard vs. intEnsive statin therapy for hypercholesteroleMic Patients with diAbetic retinopaTHY (EMPATHY) study.
Methods
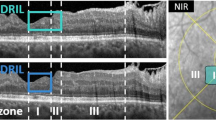
Among 5144 patients in the multicentre, prospective, randomized EMPATHY study, this substudy considered 157 patients with seven-field fundus photographs of sufficient quality taken during study enrolment and at the 3-year visit. Eighty-five and seventy-two patients received intensive and standard statin treatments, respectively, in a treat-to-target manner. The primary endpoint was a two-step change in the Early Treatment Diabetic Retinopathy Study (ETDRS) DR severity scale at 36 months. Surrogate markers included changes in hard exudates, changes in visual acuity, and additional ocular treatments during study follow-up.
Results
Intensive and standard treatment groups did not differ significantly in terms of changing two or more steps on the DR severity scale (P = 0.4380). In patients with severe DR, defined as ≥47 on the severity scale, exploratory analysis showed more frequent improvement of DR, by at least one step, with intensive vs. standard treatment (83.3% vs. 40.0%; P = 0.0346). The intensive and standard groups did not differ in changes on the hard exudates severity scale (P = 0.3460), logarithm of minimum angle of resolution visual acuity (P = 0.5500), or additional ocular treatment during follow-up.
Conclusions
Intensive and standard statin treatment may have similar effects on DR in the population of all patients with DR and hypercholesterolaemia, but intensive therapy may be more beneficial in patients with severe DR.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–83.
Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303.
Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–5.
Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–84.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–402.
Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–22.
Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97.
Group AS, Group AES, Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44.
Cholesterol Treatment Trialists Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25.
Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, et al. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164:1697–706.
Ueshima K, Itoh H, Kanazawa N, Komuro I, Nagai R, Takeuchi M, et al. Rationale and design of the standard versus intensive statin therapy for hypercholesterolemic patients with diabetic retinopathy (EMPATHY) study: a randomized controlled trial. J Atheroscler Thromb. 2016;23:976–90.
Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, et al. Intensive treat-to-target statin therapy in high-risk Japanese patients with hypercholesterolemia and diabetic retinopathy: report of a randomized study. Diabetes Care. 2018;41:1275–84.
Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, et al. Achieving LDL cholesterol target levels <1.81 mmol/L may provide extra cardiovascular protection in patients at high risk: exploratory analysis of the standard versus intensive statin therapy for patients with hypercholesterolaemia and diabetic retinopathy study. Diabetes Obes Metab. 2019;21:791–800.
Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806.
Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96.
Tauber JP, Cheng J, Gospodarowicz D. Effect of high and low density lipoproteins on proliferation of cultured bovine vascular endothelial cells. J Clin Investig. 1980;66:696–708.
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7.
Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Investig. 1995;72:638–45.
Hata Y, Miura M, Asato R, Kita T, Oba K, Kawahara S, et al. Antiangiogenic mechanisms of simvastatin in retinal endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2010;248:667–73.
Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Investig Ophthalmol Vis Sci. 2008;49:3231–8.
Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–38.
Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL 3rd. Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110:2126–33.
Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia and diabetic macular edema: a systematic review and meta-analysis. Ophthalmology. 2015;122:1820–7.
Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154:549–59.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10.
Funding
This work was supported by Shionogi & Co., Ltd.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
TM reports personal fees from Shionogi & Co., Ltd. during the conducting of the study. SK reports grants from Shionogi & Co., Ltd. during the conducting of the study. TS reports personal fees and non-financial support from Shionogi & Co., Ltd. during the conducting of the study. HI reports grants and personal fees from Shionogi & Co., Ltd. during the conducting of the study, and grants and personal fees from Takeda Pharmaceutical Company Limited, Nippon Boehringer Ingelheim Co., Ltd. Daiichi Sankyo Company, Limited, MSD K.K., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. grants from Sumitomo Dainippon Pharma Co., Ltd. Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd. Teijin Pharma Limited, Mochida Pharmaceutical Co., Ltd. Ono Pharmaceutical Co., Ltd. Chugai Pharmaceutical Co., Ltd. Eli Lilly Japan K.K., and personal fees from Nipro Corporation and SBI Pharmaceuticals Co., Ltd. outside the submitted work. IK reports personal fees from Shionogi & Co., Ltd. during the conducting of the study, grants and personal fees from Takeda Pharmaceutical Company Limited, Nippon Boehringer Ingelheim Co., Ltd. Astellas Pharma Inc., Daiichi Sankyo Company, Limited, and Otsuka Pharmaceutical Co., Ltd. and grants from MSD K.K., Shionogi & Co., Ltd. GlaxoSmithKline K.K., Sanofi K.K., Genzyme Japan K.K., Sumitomo Dainippon Pharma Co., Ltd. Mitsubishi Tanabe Pharma Corporation, and Bristol-Myers Squibb Company outside the submitted work. MT reports personal fees from Shionogi & Co., Ltd. during the conducting of the study. NY reports personal fees from Shionogi & Co., Ltd. during the conducting of the study, and personal fees from Shionogi & Co., Ltd. outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the ophthalmology substudy of EMPATHY Investigators are listed in Supplementary Data.
Supplementary information
Rights and permissions
About this article
Cite this article
Murakami, T., Kato, S., Shigeeda, T. et al. Intensive treat-to-target statin therapy and severity of diabetic retinopathy complicated by hypercholesterolaemia. Eye 35, 2221–2228 (2021). https://doi.org/10.1038/s41433-020-01202-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01202-5
This article is cited by
-
Natural products as HMG-CoA reductase inhibitors (statins) for the management of non-communicable diseases
Inflammopharmacology (2025)
-
A prediction model for worsening diabetic retinopathy after panretinal photocoagulation
Diabetology & Metabolic Syndrome (2022)
-
Diabetic retinopathy in Greece: prevalence and risk factors studied in the medical retina clinic of a Greek tertiary hospital
International Ophthalmology (2022)