Abstract
Background
Subretinal fluid is a risk factor for growth and malignant transformation of choroidal naevi, however it is unclear if this applies to subclinical fluid that is only detectable by optical coherence tomography (OCT). The objective of this study was to determine the prevalence and associations of subclinical but OCT-detectable subretinal fluid over choroidal naevi.
Methods
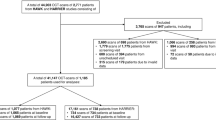
Cross-sectional study of 309 consecutive cases of choroidal naevi imaged by OCT between July 2017 to January 2019. Multicentre international study involving ten retinal specialist centres. All patients presenting to retinal specialists had routine clinical examination and OCT imaging. The prevalence of subclinical OCT-detectable subretinal fluid over choroidal naevi and its associations with other features known to predict growth and malignant transformation were noted and analysed.
Results
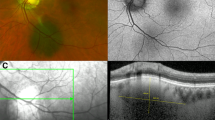
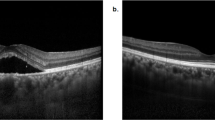
Of 309 identified consecutive cases, the mean patient age was 65 years, 89.3% of patients were Caucasian and 3.9% were Asian. The prevalence of subclinical but OCT-detectable subretinal fluid associated with choroidal naevi was 11.7% (36/309). Naevi with fluid were associated with larger basal diameters, greater thickness, presence of a halo, orange pigmentation, hyperautofluorescence, and hypodensity on B-scan ultrasonography.
Conclusion and relevance
Of choroidal naevi where subretinal fluid is not visible on clinical examination, 11.7% demonstrate subretinal fluid on OCT scans. These naevi more commonly exhibit features known to be associated with growth and transformation to melanoma. The presence of subclinical OCT-detectable fluid over choroidal naevi may assist in their risk stratification.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the blue mountains eye study. Arch Ophthalmol. 1998;116:645–50.
Ganley JP, Comstock GW. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol. 1973;76:19–25.
Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112:1784–9.
Shields CL, Cater J, Shields JA, Singh AD, Santos MCM, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000;118:360–4.
Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–7.
Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Trans Am Ophthalmological Soc. 1995;93:259–75. discussion 75-79
Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK Jr, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith Victor T Curtin Lect RETINA. 2019;39:1840.
Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–9.
Straatsma BR, Diener-West M, Caldwell R, Engstrom RE, Group* COMS. Mortality after deferral of treatment or no treatment for choroidal melanoma. J Ophthalmol. 2018;66:1395–400.
Shields CL, Mashayekhi A, Materin MA, Luo CK, Marr BP, Demirci H, et al. Optical coherence tomography of choroidal nevus in 120 patients. Retina. 2005;25:243–52.
Espinoza G, Rosenblatt B, Harbour JW. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am J Ophthalmol. 2004;137:90–5.
Jonna G, Daniels AB. Enhanced depth imaging OCT of ultrasonographically flat choroidal nevi demonstrates 5 distinct patterns. Ophthalmol Retin. 2019;3:270–7.
Kaiserman I, Kaiserman N, Pe’er J. Long term ultrasonic follow up of choroidal naevi and their transformation to melanomas. Br J Ophthalmol. 2006;90:994–8.
Shields CL, Bianciotto C, Pirondini C, Materin MA, Harmon SA, Shields JA. Autofluorescence of choroidal melanoma in 51 cases. Br J Ophthalmol. 2008;92:617–22.
Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: comparison with choroidal nevus. Arch Ophthalmol. 2012;130:850–6.
Yaghy A, Yu MD, Dalvin LA, Mazloumi M, Ferenczy SR, Shields CL. Photoreceptor morphology and correlation with subretinal fluid chronicity associated with choroidal nevus. Br J Ophthalmol. 2020;104:863–7
Yu MD, Dalvin LA, Ancona-Lezama D, Yaghy A, Ferenczy SR, Milman T, et al. Choriocapillaris compression correlates with choroidal nevus-associated subretinal fluid: OCT analysis of 3431 cases. Ophthalmology. 2020:127:863–7
Shields CL, Bianciotto C, Pirondini C, Materin MA, Harmon SA, Shields JA. Autofluorescence of orange pigment overlying small choroidal melanoma. Retina 2007;27:1107–11.
Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–98.
Mashayekhi A, Shields CL, Eagle RC, Shields JA. Vitreous and preretinal seeding after transvitreal fine needle aspiration biopsy of choroidal melanoma. Retinal Cases Brief Rep. 2019.
Chien JL, Sioufi K, Surakiatchanukul T, Shields JA, Shields CL. Choroidal nevus: a review of prevalence, features, genetics, risks, and outcomes. [Review]. Curr Opin Ophthalmol. 2017;28:228–37.
Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, et al. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology 2008;115:546–52.e2.
Shields CL, Pirondini C, Bianciotto C, Materin MA, Harmon SA, Shields JA. Autofluorescence of choroidal nevus in 64 cases. Retina 2008;28:1035–43.
International Retina Group
Adrian T. Fung1,2,3, Jay Chhablani6,7, Dinah Zur9, Matias Iglicki10, Aude Couturier11, Catharina Busch14, Marco Lupidi15, Pierre-Henry Gabrielle17, Samantha Fraser-Bell2, Achara Ampornpruet18, Patricio J. Rodriguez-Valdez19, Zafer Cebeci20, Ermete Giancipoli21,22, Voraporn Chaikitmongkol23, Mali Okada24, Inês Lains25,26,27, Anna Sala-Puigdollers28, Malgorzata Ozimek29, Matus Rehak14, Anat Loewenstein9
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the International Retina Group are listed above references.
Rights and permissions
About this article
Cite this article
Fung, A.T., Guan, R., Forlani, V. et al. Subclinical subretinal fluid detectable only by optical coherence tomography in choroidal naevi—the SON study. Eye 35, 2038–2044 (2021). https://doi.org/10.1038/s41433-020-01206-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01206-1