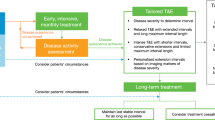
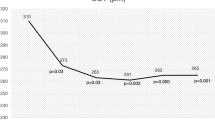
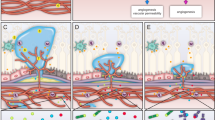
Abstract
The treatment of neovascular AMD (nAMD) has been revolutionized by the introduction of anti-vascular endothelial growth factor (VEGF) agents. Though, there is a tremendous gap between the outcomes in randomized clinical trials and real-world settings, where long-term outcomes are not as good as expected. This is due to undertreatment, i.e., fewer injection and low monitoring frequency. Treatment burden due to frequent injections remains a major limitation. Long-lasting treatments provide promising solutions for this unmet need by achieving better results with less mandatory injections. This review aims to cover the current state in this field and also discuss the mechanism of action, data from pivotal trials, and safety profile of long-acting treatments in present and future, going into details about the following agents: Brolucizumab, Faricimab, Abipicar, and Conbercept.
摘要
抗血管内皮生长因子 (VEGF) 药物的应用使新生血管性AMD (nAMD) 的治疗发生了革命性变化。但随机临床试验的结果与现实世界存在巨大差距, 临床实际的长期疗效远低于预期。这是由于治疗不足所致, 即注射次数较少以及随访频率低。其中频繁注射造成的治疗负担为主要的制约因素。而长效制剂通过减少注射次数以及获得的更好疗效, 为满足临床上述的需求提供了解决方案。本综述旨在阐述该领域的现状, 讨论长期治疗在目前和未来阶段的作用机制、重要临床试验数据和安全性, 并详细介绍以下药物: Brolucizumab、Faricimab、Abipicar和Conbercept。
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bourne RRA, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102:575–85. https://www.ncbi.nlm.nih.gov/pubmed/29545417.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–38. https://www.ncbi.nlm.nih.gov/pubmed/22559899.
Cantor LB, Rapuano C, Cioffi G. Retina and vitreous. San Francisco: American Academy of Ophthalmology; 2014.
The CATT Research Group. The CRG. Ranibizumab and bevacizumab for neovascular. N Engl J Med. 2011;364:1897–908. http://blogs.nejm.org/now/index.php/ranibizumab-and-bevacizumab-for-neovascular-age-related-macular-degeneration/2011/05/18/trackback/.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83. https://www.ncbi.nlm.nih.gov/pubmed/22306121.
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SVR, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159:915. https://doi.org/10.1016/j.ajo.2015.01.032.
Grunwald JE, Pistilli M, Daniel E, Ying G-S, Pan W, Jaffe GJ, et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124:97–104.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–6. https://www.ncbi.nlm.nih.gov/pubmed/25193672.
Ramakrishnan MS, Yu Y, VanderBeek BL. Association of visit adherence and visual acuity in patients with neovascular age-related macular degeneration: secondary analysis of the comparison of age-related macular degeneration treatment trial. JAMA Ophthalmol. 2020;138:237–42. https://doi.org/10.1001/jamaophthalmol.2019.4577.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Hykin P, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100:1623–8.
Karampelas M, Pefkianaki M, Rees A, Gill N, Kotecha A, Hamilton R, et al. Missed hospital appointments of patients receiving ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmol Ther. 2015;4:43–9.
Obeid A, Gao X, Ali FS, Aderman CM, Shahlaee A, Adam MK, et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti–vascular endothelial growth factor injections. JAMA Ophthalmol. 2018;136:1251–9. https://doi.org/10.1001/jamaophthalmol.2018.3578.
Droege KM, Muether PS, Hermann MM, Caramoy A, Viebahn U, Kirchhof B, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251:1281–4.
Brunton LL, Hilal-Dandan R, Knollmann BC Editors. In: Goodman & Gilman’s, editor. The pharmacological basis of therapeutics, 13th ed. New York, NY: McGraw-Hill Education; 2017. http://accessmedicine.mhmedical.com/content.aspx?aid=1154973599.
Sharma A, Kumar N, Parachuri N, Sharma R, Bandello F, Kuppermann BD, et al. Brolucizumab and immunogenicity. Eye. 2020;34:1726–28.
Agency EM. Assessment report; Beovu. Assess. Rep. EMA/23630/2020 Comm. Med. Prod. Hum. Use. 2019;1–6.
Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG, et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127:963–76.
Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration. Ophthalmology. 2016;123:1080–9.
Yannuzzi NA, Freund KB. Brolucizumab: evidence to date in the treatment of neovascular age-related macular degeneration. Clin Ophthalmol. 2019;ume 13:1323–9.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84.
Michael Singer MD, Effie Z, Rahman M. Update on longer-acting anti-VEGF therapies/an expansive horizon is coming into focus. Retinal Phys. 2020;17:38–41.
ASRS. American Society of Retina Specialists Research and Safety in Therapeutics Committee. 2020; p. 1. https://www.asrs.org/clinical/clinical-updates/2976/New-nbsp-ReST-Committee-Report-Summarizes-Analysis-of-Reported-Cases-of-Inflamma.
Rajan K. Update: Brolucizumab’s safety under review. Compr. Ophthalmol. Retin. https://www.aao.org/headline/brolucizumab-s-safety-under-review.
Rosenfeld PJ, Browning DJ. Is this a 737 max moment for brolucizumab? Am J Ophthalmol. 2020;216:A7–8.
Stumpp MT, Binz HK, Amstutz P. DARPins: a new generation of protein therapeutics. Drug Discov Today. 2008;13:695–701.
Stahl A, Stumpp MT, Schlegel A, Ekawardhani S, Lehrling C, Martin G, et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis. 2013;16:101–11.
Rodrigues GA, Mason M, Christie L-A, Hansen C, Hernandez LM, Burke J, et al. Functional characterization of abicipar-pegol, an anti-VEGF DARPin therapeutic that potently inhibits angiogenesis and vascular permeability. Investig Opthalmology Vis Sci. 2018;59:5836.
Moisseiev E, Loewenstein A. Abicipar pegol—a novel anti-VEGF therapy with a long duration of action. Eye. 2020;34:605–6.
Callanan D, Kunimoto D, Maturi RK, Patel SS, Staurenghi G, Wolf S, et al. Double-masked, randomized, phase 2 evaluation of abicipar pegol (an anti-VEGF DARPin therapeutic) in neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2018;34:700–9.
Kunimoto D, Yoon YH, Wykoff CC, Chang A, Khurana RN, Maturi RK, et al. Efficacy and safety of abicipar in neovascular age-related macular degeneration: 52-Week Results of Phase 3 Randomized Controlled Study. Ophthalmology. 2020;127:1331–44.
Molecular Partners AG. The MAPLE study used a modified manufacturing process and demonstrated decreased intraocular inflammation. Press RELEASE. https://www.molecularpartners.com/allergan-and-molecular-partners-announce-topline-safety-results-from-maple-study-of-abicipar-pegol/.
Jerry Helzner. DARPin fails to get approval due to inflammation issue. Retin. Physician. https://www.retinalphysician.com/issues/2020/june-2020/darpin-fails-to-get-approval-due-to-inflammation.
Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye. 2020;34:802–4.
Danzig CarlJ, Lin Hugh, Guibord Pascal, Silverman David, Ruiz CarlosQuezada, Ivo Stoilov ZH. Clinical Effects of Blocking Ang-2 and VEGF with Faricimab in the Phase 2 STAIRWAY Trial. Investig Ophthalmol Vis Sci. 2020;61:1160.
ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration (LUCERNE). https://clinicaltrials.gov/ct2/show/NCT03823300.
ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab in participants with neovascular age-related macular degeneration (TENAYA). https://clinicaltrials.gov/ct2/show/NCT03823287.
Sun X, Lu X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Dev Ther. 2015;9:2311–20.
Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration. Ophthalmology. 2014;121:1740–7.
Liu K, Song Y, Xu G, Ye J, Wu Z, Liu X, et al. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized phase 3 PHOENIX study. Am J Ophthalmol. 2019;197:156–67.
ClinicalTrials.gov. Efficacy and safety trial of conbercept intravitreal injection for neovascular age-related macular degeneration (PANDA-1). https://clinicaltrials.gov/ct2/show/NCT03577899.
ClinicalTrials.gov. Efficacy and safety trial of conbercept intravitreal injection for neovascular age-related macular degeneration (PANDA-2). https://clinicaltrials.gov/ct2/show/NCT03630952.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iglicki, M., González, D.P., Loewenstein, A. et al. Longer-acting treatments for neovascular age-related macular degeneration—present and future. Eye 35, 1111–1116 (2021). https://doi.org/10.1038/s41433-020-01309-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01309-9
This article is cited by
-
One-Year Real-World Outcomes of Aflibercept 8 mg in Treatment-Naïve Neovascular Age-Related Macular Degeneration: A Swiss Retina Research Network Report
Ophthalmology and Therapy (2026)
-
Validation of the Chinese version of the financial toxicity scale in patients with wet age-related macular degeneration
Journal of Patient-Reported Outcomes (2025)
-
Efficacy and safety of dexamethasone versus intravitreal aflibercept implants for macular edema: a systematic review and meta-analysis
European Journal of Medical Research (2025)
-
The effect of diabetes on short-term outcomes following epiretinal membrane surgery
International Ophthalmology (2024)
-
Accuracy of biomicroscopy, ultrasonography and spectral-domain OCT in detection of complete posterior vitreous detachment
BMC Ophthalmology (2023)