Abstract
Background/Objectives
To evaluate biometric changes throughout the anterior chamber during accommodation and presbyopia using single image acquisition swept-source anterior-segment optical coherence tomography (AS-OCT).
Subject/Methods
Anterior-segment images were obtained using a new swept-source AS-OCT device (ANTERION, Heidelberg Engineering) from healthy volunteers (n = 71) across two centers in this prospective observational case series. In one image acquisition, cornea through posterior lens, including the ciliary muscle on both sides of the right eye, was imaged. Subjects undertook no accommodative effort and −1, −3, and −5 D of target vergence. Two-way repeated measures ANOVA modeling was performed for ciliary muscle measurements, lens parameters, aqueous depth (AD), and pupil diameter (PD). The first ANOVA factor was accommodative stimuli, and the second factor included age and refractive status.
Results
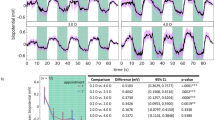
Maximum ciliary muscle thickness increased with accommodative stimuli (p < 0.001), while the distance from the scleral spur to the maximal point on the ciliary muscle and posterior ciliary muscle thickness (CMT2) decreased (p < 0.001–0.002). Older individuals showed no accommodative changes for ciliary muscle parameters, lens thickness, lens vault, PD, and AD (p = 0.07–0.32). Younger- and middle-aged eyes showed statistically significant accommodative structural alterations for these endpoints (p < 0.001–0.002), but with different patterns, including early loss of CMT2 contraction in middle-aged eyes. Within the middle-aged group, myopic eyes maintained better capacity for accommodative structural change.
Conclusions
Swept-source AS-OCT demonstrated multiple simultaneous anterior-segment biometric alterations in single acquisition images, including early loss of posterior ciliary muscle function and better maintained capacity for anterior-segment structural change in myopia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wolffsohn JS, Davies LN. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019;68:124–43.
Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–72.
Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mech Ageing Dev. 1992;62:209–21.
Lütjen-Drecoll E, Tamm E, Kaufman PL. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol. 1988;106:1591–8.
Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB, et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Investig Ophthalmol Vis Sci. 2006;47:1076–86.
Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–72.
von Helmholtz H. Mechanism of accommodation. In: Southall J, editor. Helmholtz’s treatise on physiological optics. New York: Optical Society of America; 1924. p. 382–415.
Koretz JF, Cook CA, Kaufman PL. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Investig Ophthalmol Vis Sci. 1997;38:569–78.
Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of the anteroposterior position and thickness of the aging, accommodating, phakic, and pseudophakic ciliary muscle. J Cataract Refract Surg. 2010;36:235–41.
Glasser A, Croft MA, Brumback L, Kaufman PL. Ultrasound biomicroscopy of the aging rhesus monkey ciliary region. Optom Vis Sci. 2001;78:417–24.
Esteve-Taboada JJ, Domínguez-Vicent A, Monsálvez-Romín D, Del Águila-Carrasco AJ, Montés-Micó R. Non-invasive measurements of the dynamic changes in the ciliary muscle, crystalline lens morphology, and anterior chamber during accommodation with a high-resolution OCT. Graefes Arch Clin Exp Ophthalmol. 2017;255:1385–94.
Domínguez-Vicent A, Monsálvez-Romín D, Esteve-Taboada JJ, Montés-Micó R, Ferrer-Blasco T. Effect of age in the ciliary muscle during accommodation: sectorial analysis. J Optom. 2019;12:14–21.
Richdale K, Sinnott LT, Bullimore MA, Wassenaar PA, Schmalbrock P, Kao CY, et al. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Investig Ophthalmol Vis Sci. 2013;54:1095–105.
Bailey MD. How should we measure the ciliary muscle? Investig Ophthalmol Vis Sci. 2011;52:1817–8.
Sheppard AL, Davies LN. In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Investig Ophthalmol Vis Sci. 2010;51:6882–9.
Sheppard AL, Davies LN. The effect of ageing on in vivo human ciliary muscle morphology and contractility. Investig Ophthalmol Vis Sci. 2011;52:1809–16.
Mohamed Farouk M, Naito T, Shinomiya K, Mitamura Y. Observation of ciliary body changes during accommodation using anterior OCT. J Med Investig. 2018;65:60–3.
Lossing LA, Sinnott LT, Kao CY, Richdale K, Bailey MD. Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci. 2012;89:719–26.
Kuchem MK, Sinnott LT, Kao CY, Bailey MD. Ciliary muscle thickness in anisometropia. Optom Vis Sci. 2013;90:1312–20.
Wagner S, Zrenner E, Strasser T. Emmetropes and myopes differ little in their accommodation dynamics but strongly in their ciliary muscle morphology. Vis Res. 2019;163:42–51.
Ruggeri M, de Freitas C, Williams S, Hernandez VM, Cabot F, Yesilirmak N, et al. Quantification of the ciliary muscle and crystalline lens interaction during accommodation with synchronous OCT imaging. Biomed Opt Express. 2016;7:1351–64.
Shao Y, Tao A, Jiang H, Mao X, Zhong J, Shen M, et al. Age-related changes in the anterior segment biometry during accommodation. Investig Ophthalmol Vis Sci. 2015;56:3522–30.
Huang AS, Belghith A, Dastiridou A, Chopra V, Zangwill LM, Weinreb RN. Automated circumferential construction of first-order aqueous humor outflow pathways using spectral-domain optical coherence tomography. J Biomed Opt. 2017;22:66010.
Hau SC, Papastefanou V, Shah S, Sagoo MS, Restori M, Cohen V. Evaluation of iris and iridociliary body lesions with anterior segment optical coherence tomography versus ultrasound B-scan. Br J Ophthalmol. 2015;99:81–6.
Ang M, Baskaran M, Werkmeister RM, Chua J, Schmidl D, Dos Santos VA, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132–56.
Asrani S, Sarunic M, Santiago C, Izatt J. Detailed visualization of the anterior segment using fourier-domain optical coherence tomography. Arch Ophthalmol. 2008;126:765–71.
Xie X, Corradetti G, Song A, Pardeshi A, Sultan W, Lee JY, et al. Age- and refraction-related changes in anterior segment anatomical structures measured by swept-source anterior segment OCT. PLoS ONE. 2020;15:e0240110.
Pardeshi AA, Song AE, Lazkani N, Xie X, Huang A, Xu BY. Intradevice repeatability and interdevice agreement of ocular biometric measurements: a comparison of two swept-source anterior segment OCT devices. Transl Vis Sci Technol. 2020;9:14.
Pucker AD, Sinnott LT, Kao CY, Bailey MD. Region-specific relationships between refractive error and ciliary muscle thickness in children. Investig Ophthalmol Vis Sci. 2013;54:4710–6.
Wagner S, Zrenner E, Strasser T. Ciliary muscle thickness profiles derived from optical coherence tomography images. Biomed Opt Express. 2018;9:5100–14.
Buckhurst H, Gilmartin B, Cubbidge RP, Nagra M, Logan NS. Ocular biometric correlates of ciliary muscle thickness in human myopia. Ophthalmic Physiol Opt. 2013;33:294–304.
Lockhart TE, Shi W. Effects of age on dynamic accommodation. Ergonomics. 2010;53:892–903.
Alió JL, Alió Del Barrio JL, Vega-Estrada A. Accommodative intraocular lenses: where are we and where we are going. Eye Vis. 2017;4:16.
Digre KB. Assessment of accommodation, convergence and the near response. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s clinical neuro-ophthalmology. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 726–8.
Muftuoglu O, Hosal BM, Zilelioglu G. Ciliary body thickness in unilateral high axial myopia. Eye. 2009;23:1176–81.
Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Investig Ophthalmol Vis Sci. 2008;49:4353–60.
Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005;140:324–5.
Koeppl C, Findl O, Kriechbaum K, Drexler W. Comparison of pilocarpine-induced and stimulus-driven accommodation in phakic eyes. Exp Eye Res. 2005;80:795–800.
Tabernero J, Chirre E, Hervella L, Prieto P, Artal P. The accommodative ciliary muscle function is preserved in older humans. Sci Rep. 2016;6:25551.
Acknowledgements
This study was supported by China Scholarship Council Grant (#201808110001), Capital Characteristic Clinic Project of Beijing (Z18110000171808) [XX]. AGS MAPS Award (BYX); NIH NEI R01EY030501 [ASH] and K23EY029763 [BYX]; Research to Prevent Blindness Career Development Award 2016 [ASH]; and an unrestricted grant from Research to Prevent Blindness [UCLA and USC]. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
XX was responsible for experimental design, data acquisition, data analyses, creation of figures/tables, and preparation of the manuscript. WS was responsible for data analyses, creation of figures, and preparation of the manuscript. GC was responsible for experimental design, data acquisition, data analyses, and preparation of the manuscript. JYL was responsible for data analyses and preparation of the manuscript. AS and AP were responsible for data acquisition and preparation of the manuscript. FY was responsible for statistical analyses. VC was responsible for data analyses and preparation of the manuscript. SRS, BYX, and ASH were responsible for experimental design, data analyses, and preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
ASH and BYX were loaned the ANTERION from Heidelberg Engineering for the purpose of research. ASH, SRS, and BYX received research support from Heidelberg Engineering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, X., Sultan, W., Corradetti, G. et al. Assessing accommodative presbyopic biometric changes of the entire anterior segment using single swept-source OCT image acquisitions. Eye 36, 119–128 (2022). https://doi.org/10.1038/s41433-020-01363-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-01363-3
This article is cited by
-
In vivo characterization of morphologic changes in the lens during accommodation as a function of age by use of OCT with swept-source technology
Japanese Journal of Ophthalmology (2025)