Abstract
Purpose
Single center, noninterventional cohort study to assess 10-year visual and anatomical outcomes following initiation of treatment with antivascular endothelial growth factor (anti-VEGF) agents in neovascular age-related macular degeneration (AMD) patients. Neovascular AMD patients initiated on intravitreal anti-VEGF injections in 2008–2009 and continued to be followed up for at least 10 years were included in this study.
Methods
The Moorfields OpenEyes database was searched for all patients who were initiated on anti-VEGF therapy for neovascular AMD in 2008–2009 and the visual acuity (VA) in Early Diabetic Retinopathy Study (ETDRS) letters and injection records were analyzed for those who have had at least 10-year follow-up. The spectral-domain optical coherence tomography (SD-OCT) scans, color fundus photos, and fundus fluorescein angiography (FA) were graded by two retinal physicians. The outcomes were also compared between those with good and poor VA outcomes based on pre-defined criteria. The primary end point was change in VA at 10 years; secondary outcomes included percentage with VA of 20/40 or better, 20/70 or better, VA gains and losses, anatomic outcomes and number of injections.
Results
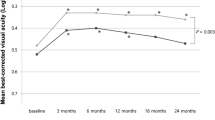
After a mean of 10.04 years after initiation of anti-VEGF therapy, the mean decline in VA from baseline was −2.1 ETDRS letters (SD 19.9, p = 0.65). One hundred eyes (67.1%) achieved a VA threshold of 20/70 or better, 33.5% achieved a VA of 20/40 or better, and 76.5% eyes maintained VA defined as a loss of less than 15 letters. Fourteen percent of study eyes had VA of 20/200 or worse and 23.5% declined by 15 letters or more. 87.5% of eyes were switched from ranibizumab to aflibercept during the course of 10 years and the eyes received a mean of 52.2 (SD 18.1) injections over 10 years. From this cohort, 87 (58.3%) eyes are having on-going treatment. On OCT, 34.9% had persistent fluid at the last visit, 6.7% patients showed new onset atrophy compared to baseline, and 43.7% had increased area of macular atrophy. The mean area of atrophy at the final visit was 4.15 mm2. Comparison between the good and worse visual outcome groups showed lower baseline VA, fovea-involving atrophy and final area of atrophy had a statistically significant negative effect on the final visual outcome (p < 0.05).
Conclusions
Regular monitoring and anti-VEGF treatment over 10 years reduce the risk of visual loss of 15 letters or more in patients with neovascular AMD. The most common cause of substantial visual decline was macular atrophy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65.e5.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–61.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–6.
Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Queré S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33:474–81.
Sunness JS, Bressler NM, Tian Y, Alexander J, Applegate CA. Measuring geographic atrophy in advanced age-related macular degeneration. Investig Ophthalmol Vis Sci. 1999;40:1761–9.
Wada I, Oshima Y, Shiose S, Kano K, Nakao S, Kaizu Y, et al. Five-year treatment outcomes following intravitreal ranibizumab injections for neovascular age-related macular degeneration in Japanese patients. Graefes Arch Clin Exp Ophthalmol. 2019;257:1411–8.
Keenan TD, Vitale S, Agrón E, Domalpally A, Antoszyk AN, Elman MJ, et al. Visual acuity outcomes after anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: age-related eye disease Study 2 Report Number 19. Ophthalmol Retina. 2019;4:3–12.
Fasler K, Moraes G, Wagner S, Kortuem KU, Chopra R, Faes L, et al. One- and two-year visual outcomes from the Moorfields age-related macular degeneration database: a retrospective cohort study and an open science resource. BMJ Open. 2019;9:e027441.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Hykin P, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100:1623–8.
Qin VL, Young J, Silva FQ, Conti FF, Singh RP. Outcomes of patients with exudative age-related macular degeneration treated with antivascular endothelial growth factor therapy for three or more years: a review of current outcomes. Retina. 2018;38:1500–8.
Kaiser PK, Singer M, Tolentino M, Vitti R, Erickson K, Saroj N, et al. Long-term safety and visual outcome of intravitreal aflibercept in neovascular age-related macular degeneration: VIEW 1 extension study. Ophthalmol Retina. 2017;1:304–13.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–67.
Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–61.
Sarraf D, London NJ, Khurana RN, Dugel PU, Gune S, Hill L, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology. 2016;123:2213–24.
Kaynak S, Kaya M, Kaya D. Is there a relationship between use of anti-vascular endothelial growth factor agents and atrophic changes in age-related macular degeneration patients? Turk J Ophthalmol. 2018;48:81–4.
Lee AY, Lee CS, Butt T, Xing W, Johnston RL, Chakravarthy U, et al. UK AMD EMR USERS GROUP REPORT V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol. 2015;99:1045–50.
Talks JS, Lotery AJ, Ghanchi F, Sivaprasad S, Johnston RL, Patel N, et al. First-year visual acuity outcomes of providing aflibercept according to the view study protocol for age-related macular degeneration. Ophthalmology. 2016;123:337–43.
Acknowledgements
We would like to thank the Moorfields Medical Retina Group for their contribution and collation of patients. The research was supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SS reported receiving research grants from Novartis, Bayer, Allergan, Roche, Boehringer Ingelheim, and Optos Plc, travel grants from Novartis and Bayer, speaker fees from Novartis, Bayer, and Optos Plc, and attending advisory board meetings for Novartis, Bayer, Allergan, Roche, Boehringer Ingelheim, Optos Plc, and Heidelberg Engineering. PH reported receiving research grants from Novartis Allergan and Bayer, travel grants from Novartis Allergan and Bayer, speaker fees from Novartis Allergan and Bayer, and attending advisory board meetings for Novartis, Bayer, and Allergan. LN reported receiving speaker fees from Allergan. RH reports personal fees from Bayer, Novartis, Allergan and Ellex. PAK has received speaker fees from Heidelberg Engineering, Topcon, Carl Zeiss, Meditec, Haag-Streit, Allergan, Novartis and Bayer. He has served on advisory boards for Novartis and Bayer and has been external consultant for DeepMind and Optos. He is supported by a UK National Institute for Health Research (NIHR) Clinician Scientist Award (NIHR-CS—2014-12-23). SC, CA, DM, HK, and BP have no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandra, S., Arpa, C., Menon, D. et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye 34, 1888–1896 (2020). https://doi.org/10.1038/s41433-020-0764-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-0764-9
This article is cited by
-
Long-term observational data on neovascular age-related macular degeneration: a 10-year follow-up study
International Ophthalmology (2025)
-
Imaging biomarkers and artificial intelligence for diagnosis, prediction, and therapy of macular fibrosis in age-related macular degeneration: Narrative review and future directions
Graefe's Archive for Clinical and Experimental Ophthalmology (2025)
-
AMD-SD: An Optical Coherence Tomography Image Dataset for wet AMD Lesions Segmentation
Scientific Data (2024)
-
Brolucizumab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: Ophthalmology and Diabetology Treatment Aspects
Ophthalmology and Therapy (2023)
-
Final 4-year results of the RAINBOW real-world study: intravitreal aflibercept dosing regimens in France in treatment-naïve patients with neovascular age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)