Abstract
Objective
To evaluate the risk of stroke associated with intravitreal ranibizumab in age-related macular degeneration (AMD).
Methods
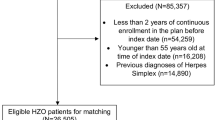
A nationwide retrospective case-crossover study was performed using data from the Korean National Health Insurance Service (KNHIS) database, which included patients with exudative AMD in South Korea (n = 41,860). The index date was the date of hospitalization for stroke. We defined the case period as 60 days and four control periods before the index date. A pharmacy prescription database was searched for ranibizumab use during the case and control periods. We calculated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) with a conditional logistic regression model.
Results
A total of 865 patients with AMD and incident stroke were included. Of all the patients, 12.02% had been treated during the preceding 60-day case period, compared with 9.25–10.29% during control periods. The adjusted OR of stroke associated with intravitreal ranibizumab during the case period was 1.285 (95% CI 0.979–1.686) (p = 0.07). In the subgroup analysis, the risk of hemorrhagic stroke had an OR of 2.252 (95% CI 1.068–4.749, p = 0.033). Further analyses based on patient gender, age, and different risk periods of 15 and 30 days yielded no increase in the risk of stroke associated with intravitreal ranibizumab.
Conclusions
This case-crossover analysis revealed no evidence of increased risk of hospitalization for stroke within 60 days of intravitreal ranibizumab injection in AMD patients. A secondary analysis indicated the possibility of an increased risk of hemorrhagic stroke, with borderline significance. Further research is needed regarding the underlying biological mechanisms and drug safety.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Tunon J, Ruiz-Moreno JM, Martin-Ventura JL, Blanco-Colio LM, Lorenzo O, Egido J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009;54:339–48.
Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116:362.
Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–9.
Ueta T, Noda Y, Toyama T, Yamaguchi T, Amano S. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014;121:2193–203 e1–7.
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116:1731–9.
Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–24.
Pratt NL, Ramsay EN, Kemp A, Kalisch-Ellett LM, Shakib S, Caughey GE, et al. Ranibizumab and risk of hospitalisation for ischaemic stroke and myocardial infarction in patients with age-related macular degeneration: a self-controlled case-series analysis. Drug Saf. 2014;37:1021–7.
Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina. 2012;32:1821–8.
Zarbin MA, Francom S, Grzeschik S, Tuomi L, Haskova Z, Macfadden W, et al. Systemic safety in ranibizumab-treated patients with neovascular age-related macular degeneration: a patient-level pooled analysis. Ophthalmol Retin. 2018;2:1087–96.
Dugel PU, Singh N, Francom S, Cantrell RA, Grzeschik SM, Fung AE. The systemic safety of ranibizumab in patients 85 years and older with neovascular age-related macular degeneration. Ophthalmol Retin. 2018;2:667–75.
Campbell RJ, Bell CM, Paterson JM, Bronskill SE, Moineddin R, Whitehead M, et al. Stroke rates after introduction of vascular endothelial growth factor inhibitors for macular degeneration: a time series analysis. Ophthalmology. 2012;119:1604–8.
Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ. 2012;345:e4203.
Rim TH, Lee CS, Lee SC, Kim DW, Kim SS. Intravitreal ranibizumab therapy for neovascular age-related macular degeneration and the risk of stroke: a national sample cohort study. Retina. 2016;36:2166–74.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95.
Lee WA, Cheng CL, Lee CH, Kao Yang YH, Lin SJ, Hsieh CY. Risks of newly onset hemorrhagic stroke in patients with neovascular age-related macular degeneration. Pharmacoepidemiol Drug Saf. 2017;26:1277–85.
Wieberdink RG, Ho L, Ikram MK, Koudstaal PJ, Hofman A, de Jong PT, et al. Age-related macular degeneration and the risk of stroke: the Rotterdam study. Stroke. 2011;42:2138–42.
Kim H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean heart study (1). Korean Circ J. 2012;42:10–5.
Park JK, Kim KS, Kim CB, Lee TY, Lee KS, Lee DH, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33:76–82.
Acknowledgements
This work was supported by a National Health Insurance Ilsan Hospital grant (NHIMC 2015-02-015). This study used data from the NHIS-NCS 2002–2013 (NHIS-2015-1-070), which was released by the KNHIS. The authors alone are responsible for the content and writing of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, J., Kim, D.W., Kim, D.H. et al. Risk of stroke associated with intravitreal ranibizumab injections in age-related macular degeneration: a nationwide case-crossover study. Eye 35, 601–607 (2021). https://doi.org/10.1038/s41433-020-0911-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-0911-3
This article is cited by
-
Risk analysis for patients with arterial thromboembolic events after intravitreal ranibizumab or aflibercept injections
Scientific Reports (2023)
-
Risk of Myocardial Infarction, Stroke, or Death in New Users of Intravitreal Aflibercept Versus Ranibizumab: A Nationwide Cohort Study
Ophthalmology and Therapy (2022)
-
Effect Modification by Indication to the Risks of Major Thromboembolic Adverse Events in Patients Receiving Intravitreal Anti-Vascular Endothelial Growth Factor Treatment: A Population-Based Retrospective Cohort Study
BioDrugs (2022)
-
Comparative Risk of Arterial Thromboembolic Events Between Aflibercept and Ranibizumab in Patients with Maculopathy: A Population-Based Retrospective Cohort Study
BioDrugs (2021)