Abstract
Objective
To develop a fully automated method of retinal pigmented epithelium (RPE) cells detection, segmentation and analysis based on in vivo cellular resolution images obtained with the transscleral optical phase imaging method (TOPI).
Methods
Fourteen TOPI–RPE images from 11 healthy individuals were analysed. The developed image processing method encompassed image filtering and normalisation, detection and removal of blood vessels, cell detection and cell membrane segmentation. The produced measures were cellular density of RPE layer, cell area, number of neighbouring cells, eccentricity, circularity and solidity. In addition, we proposed coefficient of variation (CV) of RPE cellular membrane (CMDCV) and the solidity of the RPE cell membrane-shape as new metrics for the assessment of RPE single cells.
Results
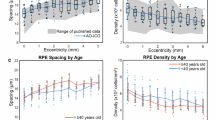
The observed median cellular density of the RPE layer was 3743 cells/µm2 (interquartile rate (IQR) 1687), with a median observed RPE cell area of 193 µm2 (IQR 141). The mean number of neighbouring cells was 5.22 (standard deviation (SD) 0.05) per RPE cell. The mean RPE cell eccentricity was 0.67 (SD 0.02), median circularity 0.83 (IQR 0.01), and median solidity 0.92 (IQR 0.00). The median CMDCV was 0.19 (IQR 0.02). The method is characterised by a median image processing and analysis time of 48 sec (IQR 12) per image.
Conclusions
The present study provides the first fully automated quantitative assessment of human RPE single cells in vivo. The method provides a baseline for future research in the field of clinical ophthalmology, enabling characterisation and diagnostics of retinal diseases at the single-cell level.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The image processing codes and datasets generated and or analysed during the current study are available from the corresponding author on reasonable request and subject to the ethical approvals in place and material transfer agreements.
References
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017;5:e1221–34.
Laforest T, Künzi M, Kowalczuk L, Carpentras D, Behar-Cohen F, Moser C. Transscleral optical phase imaging of the human retina. Nature Photonics. 2020.
Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81.
Korkka I, Viheriala T, Juuti-Uusitalo K, Uusitalo-Jarvinen H, Skottman H, Hyttinen J, et al. Functional voltage-gated calcium channels are present in human embryonic stem cell-derived retinal pigment epithelium. Stem Cells Transl Med. 2019;8:179–93.
Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31.
Chrenek MA, Dalal N, Gardner C, Grossniklaus H, Jiang Y, Boatright JH, et al. Analysis of the RPE sheet in the rd10 retinal degeneration model. Adv Exp Med Biol. 2012;723:641–7.
Bhatia SK, Rashid A, Chrenek MA, Zhang Q, Bruce BB, Klein M, et al. Analysis of RPE morphometry in human eyes. Mol Vis. 2016;22:898–916.
Jacques SL, Kelly MW, Lin CP. Microcavitation and cell injury in RPE cells following short-pulsed laser irradiation. Laser-Tissue Interact. 1997;VIII:174–9.
Laforest T, Carpentras D, Künzi M, Kowalczuk L, Behar-Cohen F, Moser C. A new microscopy for imaging retinal cells. arXiv e-prints [Internet]. 2017 December 01, 2017:[arXiv:1712.08472 p.]. https://ui.adsabs.harvard.edu/abs/2017arXiv171208472L.
Taylor DJ, Hobby AE, Binns AM, Crabb DP. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open. 2016;6: e011504
Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15(Pt 3):384–9.
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16.
Chen Q, Leng T, Zheng L, Kutzscher L, Ma J, de Sisternes L, et al. Automated drusen segmentation and quantification in SD-OCT images. Med Image Anal. 2013;17:1058–72.
Chiu SJ, Izatt JA, O’Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Investig Ophthalmol Vis Sci. 2012;53:53–61.
Srinivasan PP, Heflin SJ, Izatt JA, Arshavsky VY, Farsiu S. Automatic segmentation of up to ten layer boundaries in SD-OCT images of the mouse retina with and without missing layers due to pathology. Biomed Opt Express 2014;5:348–65.
Fischer J, Otto T, Delori F, Pace L, Staurenghi G. Scanning Laser Ophthalmoscopy (SLO). In: Bille JF, editor. High Resolution Imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham (CH): Springer; 2019. p. 35–57.
Zhang L, Song W, Shao D, Zhang S, Desai M, Ness S, et al. Volumetric fluorescence retinal imaging in vivo over a 30-degree field of view by oblique scanning laser ophthalmoscopy (oSLO). Biomed Opt Express 2018;9:25–40.
Hu Z, Medioni GG, Hernandez M, Sadda SR. Automated segmentation of geographic atrophy in fundus autofluorescence images using supervised pixel classification. J Med Imaging (Bellingham) 2015;2:014501.
Marin D, Gegundez-Arias ME, Suero A, Bravo JM. Obtaining optic disc center and pixel region by automatic thresholding methods on morphologically processed fundus images. Comput Methods Prog Biomed. 2015;118:173–85.
Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E. Anatomy of the eye and orbit. The Eye 2016;1–102.e2.
Rashid A, Bhatia SK, Mazzitello KI, Chrenek MA, Zhang Q, Boatright JH, et al. RPE cell and sheet properties in normal and diseased eyes. Adv Exp Med Biol. 2016;854:757–63.
He K, Sun J, Tang X. Single image haze removal using dark channel prior. IEEE Trans Pattern Anal Mach Intell. 2011;33:2341–53.
Niu X-m, Lu Z-m, Sun S-h. Digital image watermarking based on multiresolution decomposition. Electron Lett. 2000;36:1108.
Seung-mok O, McClellan JH. Multiresolution imaging with quadtree backprojection. Conference Record of Thirty-Fifth Asilomar Conference on Signals, Systems and Computers (CatNo01CH37256), Pacific Grove, CA, USA, 2001. p. 105–9.
Seongman K, Seunghyeon R, Jun Geun J, Kyu Tae P. Interframe coding using two-stage variable block-size multiresolution motion estimation and wavelet decomposition. IEEE Trans Circuits Syst Video Technol. 1998;8:399–410.
Shusterman E, Feder M. Image compression via improved quadtree decomposition algorithms. IEEE Trans Image Process 1994;3:207–15.
Hasanujjaman, Banerjee A, Biswas U, Naskar MK. Fractal image compression of an atomic image using quadtree decomposition. Devices for Integrated Circuit (DevIC), Kalyani, India, 2019. p. 501–4.
Albarrak A, Coenen F, Zheng Y. Dictionary learning-based volumetric image classification for the diagnosis of age-related macular degeneration. Machine learning and data mining in pattern recognition. Lecture notes in computer science; Springer, Cham, 2014;8556:272–84.
Sun Y, Li S, Sun Z. Fully automated macular pathology detection in retina optical coherence tomography images using sparse coding and dictionary learning. J Biomed Opt. 2017;22:16012.
Abu Khamidakh AE, Dos Santos FC, Skottman H, Juuti-Uusitalo K, Hyttinen J. Semi-automatic method for Ca(2+) imaging data analysis of maturing human embryonic stem cells-derived retinal pigment epithelium. Ann Biomed Eng. 2016;44:3408–20.
Dos Santos FL, Joutsen A, Paci M, Salenius J, Eskola H. Automatic detection of carotid arteries in computed tomography angiography: a proof of concept protocol. Int J Cardiovasc Imaging 2016;32:1299–310.
Caetano Dos Santos FL, Kolasa M, Terada M, Salenius J, Eskola H, Paci M. VASIM: an automated tool for the quantification of carotid atherosclerosis by computed tomography angiography. Int J Cardiovasc Imaging. 2019;35:1149–59
Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 1998;7:27–41.
Tseng Q, Duchemin-Pelletier E, Deshiere A, Balland M, Guillou H, Filhol O, et al. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc Natl Acad Sci USA 2012;109:1506–11.
Rossi EA, Rangel-Fonseca P, Parkins K, Fischer W, Latchney LR, Folwell MA, et al. In vivo imaging of retinal pigment epithelium cells in age related macular degeneration. Biomed Opt Expr 2013;4:2527–39.
Rangel-Fonseca P, Gomez-Vieyra A, Malacara-Hernandez D, Wilson MC, Williams DR, Rossi EA. Automated segmentation of retinal pigment epithelium cells in fluorescence adaptive optics images. J Opt Soc Am A Opt Image Sci Vis. 2013;30:2595–604.
Liu Z, Kocaoglu OP, Miller DT. 3D imaging of retinal pigment epithelial cells in the living human retina. Investig Ophthalmol Vis Sci. 2016;57:OCT533–43.
Grieve K, Gofas-Salas E, Ferguson RD, Sahel JA, Paques M, Rossi EA. In vivo near-infrared autofluorescence imaging of retinal pigment epithelial cells with 757 nm excitation. Biomed Opt Expr. 2018;9:5946–61.
Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am J Ophthalmol. 1996;121:181–9.
Besch D, Jagle H, Scholl HP, Seeliger MW, Zrenner E. Inherited multifocal RPE-diseases: mechanisms for local dysfunction in global retinoid cycle gene defects. Vis Res. 2003;43:3095–108.
Boatright JH, Dalal N, Chrenek MA, Gardner C, Ziesel A, Jiang Y, et al. Methodologies for analysis of patterning in the mouse RPE sheet. Mol Vis. 2015;21:40–60.
Shao Z, Wang H, Zhou X, Guo B, Gao X, Xiao Z, et al. Spontaneous generation of a novel foetal human retinal pigment epithelium (RPE) cell line available for investigation on phagocytosis and morphogenesis. Cell Prolif. 2017;50:e12386.
Watzke RC, Soldevilla JD, Trune DR. Morphometric analysis of human retinal pigment epithelium: correlation with age and location. Curr Eye Res. 1993;12:133–42.
Acknowledgements
The authors thank Dr. Irmela Mantel together with the team of the centre for clinical investigation for their time spent in performing the ophthalmologic checks for our study on healthy participants.
Funding
In addition to the research partners, this study was supported by the following programs: Enable program of the Technology Transfer Office at EPFL, EPFL Innogrant, Bridge proof of concept (InnoSuisse and SNSF), Gebert Rüf Stiftung foundation (GRS-052/17) and EIT Health Innovation by idea (19323-ASSESS). The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
FLCS: Study concept and design, analysis and interpretation of data, drafting the paper, critical revision of the paper for important intellectual content, statistical analysis, and technical and material support; TL: Acquisition of data and critical revision of the paper for important intellectual content; MK: Acquisition of data and critical revision of the paper for important intellectual content; LK: Critical revision of the paper for important intellectual content; FBC: Critical revision of the paper for important intellectual content and study supervision; CM: Critical revision of the paper for important intellectual content, obtained funding and study supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors TL, MK, FBC and CM are involved in a company (EarlySight SA) aiming at commercialising the TOPI technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caetano dos Santos, F.L., Laforest, T., Künzi, M. et al. Fully automated detection, segmentation, and analysis of in vivo RPE single cells. Eye 35, 1473–1481 (2021). https://doi.org/10.1038/s41433-020-1036-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-1036-4