Abstract
Objective
To assess the course of neurodegeneration based on retinal layer thickness and integrity analysis in diabetic patients without retinopathy and to evaluate its association with inner retinal reflectivity.
Methods
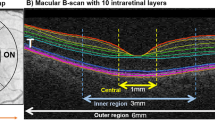
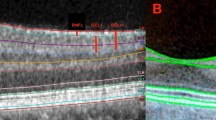
This retrospective case–control study included 80 eyes of 80 patients with DM without retinopathy and 40 eyes of 40 healthy subjects with a follow-up of ≥1 year. SD-OCT was used for assessment of retinal reflectivity and macular layer thicknesses. Optical intensity ratios (OIRs) were defined as the mean OCT reflectivity of ganglion cell and inner nuclear layer to the mean reflectivity of RPE.
Results
After Bonferroni correction, thinning in pericentral, superior and nasal sectors in total retina, superior ganglion cell, pericentral and nasal inner plexiform, and superior inner retinal layers, as well as thickening in inferior and pericentral outer plexiform layer remained significant in the study group (p < 0.0125). Ganglion cell layer OIR significantly correlated with the changes in superior retina (r = 0.278, p = 0.013), central inner retina (r = 0.247, p = 0.027), and pericentral retinal thickness (r = 0.240, p = 0.032), and no eyes had disruption of retinal layers in the study group initially or finally.
Conclusion
Ganglion cell layer reflectivity significantly correlated with the amount of pericentral retinal thinning during the time course in the diabetic group, which was more prominent in the inner retinal layers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–56.
Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol. 1999;14:223–32.
Kim K, Kim ES, Kim DG, Yu SY. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: longitudinal study using OCT angiography. Acta Diabetol. 2019;56:1275–82.
De Clerck EEB, Schouten JSAG, Berendschot TTJM, Goezinne F, Dagnelie PC, Schaper NC, et al. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht Study. Acta Ophthalmol. 2018;96:174–82.
Chhablani J, Sharma A, Goud A, Peguda HK, Rao HL, Begum VU, et al. Neurodegeneration in Type 2 diabetes: evidence from spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56:6333–8.
Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–63. 28
Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–42.
Carrasco E, Hernández C, de Torres I, Farrés J, Simó R. Lowered cortistatin expression is an early event in the human diabetic retina and is associated with apoptosis and glial activation. Mol Vis. 2008;15:1496–502. 14
Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, et al. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye (Lond). 2009;23:1824–30.
Laron M, Bearse MA Jr, Bronson-Castain K, Jonasdottir S, King-Hooper B, Barez S, et al. Interocular symmetry of abnormal multifocal electroretinograms in adolescents with diabetes and no retinopathy. Invest Ophthalmol Vis Sci. 2012;53:316–21. 25
Tyrberg M, Lindblad U, Melander A, Lövestam-Adrian M, Ponjavic V, Andréasson S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc Ophthalmol. 2011;123:193–8.
Garcia-Martin E, Bambo MP, Marques ML, Satue M, Otin S, Larrosa JM, et al. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 2016;94:e454–9.
Bittersohl D, Stemplewitz B, Keserü M, Buhmann C, Richard G, Hassenstein A. Detection of retinal changes in idiopathic Parkinson’s disease using high-resolution optical coherence tomography and heidelberg retina tomography. Acta Ophthalmol. 2015;93:e578–84.
van de Kreeke JA, Darma S, Chan Pin Yin JMPL, Tan HS, Abramoff MD, JWR Twisk, et al. The spatial relation of diabetic retinal neurodegeneration with diabetic retinopathy. PLoS One. 2020;15:e0231552. 16
Pinilla I, Idoipe M, Perdices L, Sanchez-Cano A, Acha J, Lopez-Galvez MI, et al. Changes in total and inner retinal thicknesses in type 1 diabetes with no retinopathy after 8 years of follow-up. Retina. 2020;40:1379–86.
Furashova O, Matthé E. Retinal changes in different grades of retinal artery occlusion: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2017;58:5209–16. 1
Browning DJ, Punjabi OS, Lee C. Assessment of ischemia in acute central retinal vein occlusion from inner retinal reflectivity on spectral domain optical coherence tomography. Clin Ophthalmol. 2016;22:71–79.
Mehta N, Lavinsky F, Gattoussi S, Seiler M, Wald KJ, Ishikawa H, et al. Increased inner retinal layer reflectivity in eyes with acute crvo correlates with worse visual outcomes at 12 months. Invest Ophthalmol Vis Sci. 2018;59:3503–10. 2
Toprak I, Yildirim C, Yaylali V. Impaired photoreceptor inner segment ellipsoid layer reflectivity in mild diabetic retinopathy. Can J Ophthalmol. 2015;50:438–41.
Lim HB, Shin YI, Lee MW, Park GS, Kim JY. Longitudinal changes in the peripapillary retinal nerve fiber layer thickness of patients with type 2 diabetes. JAMA Ophthalmol. 2019;137:1125–32.
Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci USA. 2016;113:E2655–64. 10
Eliwa TF, Hussein MA, Zaki MA, Raslan OA. Outer retinal layer thickness as good visual predictor in patients with diabetic macular edema. Retina. 2018;38:805–11.
Campos A, Campos EJ, do Carmo A, Caramelo F, Martins J, Sousa JP, et al. Evaluation of markers of outcome in real-world treatment of diabetic macular edema. Eye Vis (Lond). 2018;5:27. 11
Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16.
Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136:202–8. 1
Wanek J, Blair NP, Chau FY, Lim JI, Leiderman YI, Shahidi M. Alterations in retinal layer thickness and reflectance at different stages of diabetic retinopathy by en face optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57:OCT341–7. 1
Gao W, Tátrai E, Ölvedy V, Varga B, Laurik L, Somogyi A, et al. Investigation of changes in thickness and reflectivity from layered retinal structures of healthy and diabetic eyes with optical coherence tomography. J Biomed Sci Eng. 2011;4:657–65.
Pelosini L, Hull CC, Boyce JF, McHugh D, Stanford MR, Marshall J. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52:2741–8. 25
Joltikov KA, Sesi CA, de Castro VM, Davila JR, Anand R, Khan SM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:5481–6. 1
Kim K, Kim ES, Yu SY. Longitudinal relationship between retinal diabetic neurodegeneration and progression of diabetic retinopathy in patients with type 2 diabetes. Am J Ophthalmol. 2018;196:165–72.
Acknowledgements
This study was presented in part (poster presentation) at the 20th EURETINA (Virtual Congress, October 2020).
Author information
Authors and Affiliations
Contributions
ENC was responsible for the conception and design of the study, and was a major contributor in data analysis, interpretation and drafting of the manuscript. OP, HSA and GP collected and interpreted the data and contributed in writing the manuscript. All authors approved the final version and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cetin, E.N., Parca, O., Akkaya, H.S. et al. Association of inner retinal reflectivity with qualitative and quantitative changes in retinal layers over time in diabetic eyes without retinopathy. Eye 36, 1253–1260 (2022). https://doi.org/10.1038/s41433-021-01607-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01607-w