Abstract
Purpose
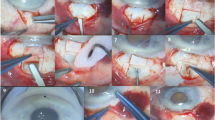
To evaluate the 12-month outcomes of a novel posterior small incision sub-tenon ab interno technique of XEN stent insertion (‘Semi-open’).
Method
Consecutive eyes underwent XEN stent insertion with the Semi-open technique by two surgeons between 1st July 2018 and 30th September 2019. All cases received subconjunctival injection of 0.1 mL of mitomycin C 0.2 mg/mL. Eyes with primary open angle glaucoma (OAG), secondary OAG or pseudophakic primary angle closure glaucoma (PACG) were included. Exclusion criteria were phakic PACG, uveitic or neovascular glaucoma and postoperative follow-up <12 months. Primary outcomes were defined by World Glaucoma Association guidelines. Secondary outcomes included change in glaucoma medications, needling rates and complications.
Results
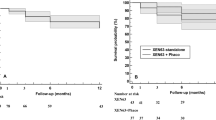
We included 37 consecutive eyes of 35 patients with primary OAG (n = 30), secondary OAG (n = 6) and pseudophakic PACG (n = 1). Thirty-one eyes (84%) received a standalone XEN implantation and 6 (16%) underwent XEN implantation combined with phacoemulsification. The IOP reduced from 19.6 ± 6.0 mmHg preoperatively to 11.2 ± 2.6 mmHg at 12 months (P < 0.01). The number of glaucoma agents reduced from 3.49 ± 1.14 preoperatively to 1.57 ± 1.36 at 12 months. At 12 months, qualified success was 97.3% and complete success was 32%, with one case requiring trabeculectomy. Needling was required in 19% of cases over the 12 month follow up. Complications included 19 cases of transient hypotony and 7 cases of transient choroidal effusion. There were no cases of exposure, bleb leak or bleb-related infection.
Conclusion
Semi-open XEN technique achieves high surgical success rate in the medium-term with relatively low post-operative bleb needling rate.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gedde SJ, Schiffman JC, Feuer WJ, Herndon L, Brandt J, Budenz D, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803.e2.
De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38:1129–34.
Fea A, Durr G, Marolo P, Malinverni L, Economou M, Ahmed I. XEN gel stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clin Ophthalmol. 2020;14:1805–32.
Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124:1579–88.
Lenzhofer M, Strohmaier C, Hohensinn M, Hitzl W, Sperl P, Gerner M, et al. Longitudinal bleb morphology in anterior segment OCT after minimally invasive transscleral ab interno glaucoma gel microstent implantation. Acta Ophthalmol. 2019;97:e231–7.
Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K, Stalmans I, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257:983–96.
Theilig T, Rehak M, Busch C, Bormann C, Schargus M, Unterlauft J. Comparing the efficacy of trabeculectomy and XEN gel microstent implantation for the treatment of primary open-angle glaucoma: a retrospective monocentric comparative cohort study. Sci Rep. 2020;10:19337.
Lee RMH, Bouremel Y, Eames I, Brocchini S, Khaw PT. The implications of an ab interno versus ab externo surgical approach on outflow resistance of a subconjunctival drainage device for intraocular pressure control. Transl Vis Sci Technol. 2019;8:58.
Shaarawy TM, Sherwood MB, Grehn F, ed. WGA guidelines on design and reporting of glaucoma surgical trials. Amsterdam: Kugler Publications; 2008.
Vera V, Ahmed IIK, Stalmans I, Reitsamer H. Gel stent implantation—recommendations for preoperative assessment, surgical technique, and postoperative management. US Ophthalmic Rev. 2018;11:38–46.
Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, Gargallo-Benedicto A, Osorio-Alayo V, Barreiro-Rego A, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91:415–21.
Heidinger A, Schwab C, Lindner E, Riedl R, Mossböck G. A Retrospective Study of 199 XEN45 Stent Implantations From 2014 to 2016. J Glaucoma. 2019;28:75–9.
Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery: 1-year results. J Glaucoma. 2017;26:1130–6.
Grover DS, Flynn WJ, Bashford KP, Lewis RA, Duh YJ, Nangia RS, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36.
Tan SZ, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye. 2018;32:324–32.
Mansouri K, Gillmann K, Rao HL, Guidotti J, Mermoud A. Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J Glaucoma. 2018;27:869–73.
Gedde SJ, Feuer WJ, Lim KS, Barton K, Goyal S, Ahmed IIK, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 3 years of follow-up. Ophthalmology. 2020;127:333–45.
Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013;120:2532–9.
Yu DY, Morgan WH, Sun X, Su EN, Cringle SJ, Yu PK, et al. The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res. 2009;28:303–28.
Sng CC, Wang J, Hau S, Htoon HM, Barton K. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2017;46:339–34.
Arnould L, Theillac V, Moran S. Recurrent exposure of XEN gel stent implant and conjunctival erosion. J Glaucoma. 2019;28:e37–40.
Karimi A, Lindfield D, Turnbull A, Dimitriou C, Bhatia B, Radwan M, et al. A multi-centre interventional case series of 259 ab-interno XEN gel implants for glaucoma, with and without combined cataract surgery. Eye. 2019;33:469–77.
Arnljots TS, Economou MA. XEN gel stent exposure 7mo after primary implantation: a case report. Int J Ophthalmol. 2019;12:689–91.
Lim R, Sethi M, Morley AMS. Ophthalmic manifestations of xeroderma pigmentosum: a perspective from the United Kingdom. Ophthalmology. 2017;124:1652–61.
Buffault J, Baudouin C, Labbé A. Is the XEN® Gel Stent really minimally invasive? Am J Ophthalmol Case Rep. 2020;19:100850.
Author information
Authors and Affiliations
Contributions
Kong Yu Xiang George contributed by providing cases for the case series, data analysis and manuscript preparation. Ghee Soon Ang contributed by providing cases for case series and manuscript proof. Young Chung contributed to data entry and manuscript proof. FRB!Glaucoma Save Sight Registries (http://savesightregistries.org/) has provided support for data aggregation and summary analysis.
Corresponding author
Ethics declarations
Competing interests
Kong Yu Xiang George receives continuing medical education funding from Allergan as part of the International Glaucoma Panel programme. Kong Yu Xiang George also act as a medical consultant for Allergan/Abbvie (China). Young Chung and Ghee Soon Ang have no conflicts of interest to disclose in relation to this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Précis The Semi-open sub-tenon XEN stent technique achieved high qualified surgical success rate, absence of bleb leak and low needling rates at 12 months.
Rights and permissions
About this article
Cite this article
Kong, Y.X.G., Chung, I.Y. & Ang, G.S. Outcomes of XEN45 gel stent using posterior small incision sub-tenon ab interno insertion (Semi-open) technique. Eye 36, 1456–1460 (2022). https://doi.org/10.1038/s41433-021-01635-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01635-6
This article is cited by
-
Update on XEN Gel Stent: A Narrative Review on Indications, Surgical Technique, and Postoperative Management
Ophthalmology and Therapy (2026)