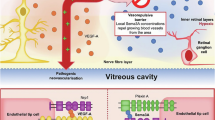
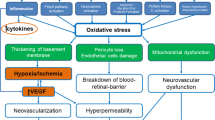
Abstract
Diabetic retinopathy is a major cause of vision loss worldwide and areas of retinal non-perfusion (RNP) are a key pathologic feature of the vascular component of diabetic retinopathy. While there is a need for a more complete understanding of the natural history of RNP development and progression, overall, increasing RNP has been closely linked with worsening DR severity. Both traditional and novel approaches to quantitative image assessment are being explored to advance our understanding of the vascular, physiologic and functional changes associated with progressive RNP. Retinal ischemia secondary to RNP leads to tissue hypoxia and changes in the expression of a host of signalling molecules. Current anti-vascular endothelial growth factor and steroid pharmaceutical agents appear to be unable to reperuse areas of RNP, but may be able to slow the progressive longitudinal accumulation of RNP with regular retreatments. There remains a tremendous unmet need for pharmacotherapies that can slow RNP progression and ultimately reperfuse areas of the non-perfused retina. Towards this end, novel targets including the semaphorin family are being investigated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98:823–33. https://doi.org/10.1016/S0161-6420(13)38014-2.
Reddy RK, Pieramici DJ, Gune S, Ghanekar A, Lu N, Quezada-Ruiz C, et al. Efficacy of ranibizumab in eyes with diabetic macular edema and macular nonperfusion in RIDE and RISE. Ophthalmology. 2018;125:1568–74. https://doi.org/10.1016/j.ophtha.2018.04.002.
Kim YJ, Yeo JH, Son G, Kang H, Sung YS, Lee JY, et al. Efficacy of intravitreal aflibercept injection for Improvement of retinal nonperfusion in diabetic retinopathy (AFFINITY study). BMJ Open Diab Res Care. 2020;8:e001616. https://doi.org/10.1136/bmjdrc-2020-001616.
de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70. https://doi.org/10.1097/IAE.0000000000000882.
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017;58:190. https://doi.org/10.1167/iovs.16-20531.
Silva PS, Dela Cruz AJ, Ledesma MG, van Hemert J, Radwan A, Cavallerano JD, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465–72. https://doi.org/10.1016/j.ophtha.2015.07.034.
Sędziak-Marcinek B, Teper S, Chełmecka E, Wylęgała A, Marcinek M, Bas M, et al. Diabetic macular edema treatment with bevacizumab does not depend on the retinal nonperfusion presence. J Diabetes Res. 2021;2021:1–15. https://doi.org/10.1155/2021/6620122.
Ehlers JP, Jiang AC, Boss JD, Hu M, Figueiredo N, Babiuch A, et al. Quantitative ultra-widefield angiography and diabetic retinopathy severity: an assessment of panretinal leakage index, ischemic index and microaneurysm count. Ophthalmology. 2019;126:1527–32. https://doi.org/10.1016/j.ophtha.2019.05.034.
Antaki F, Coussa RG, Mikhail M, Archambault C, Lederer DE. The prognostic value of peripheral retinal nonperfusion in diabetic retinopathy using ultra-widefield fluorescein angiography. Graefes Arch Clin Exp Ophthalmol. 2020;258:2681–90. https://doi.org/10.1007/s00417-020-04847-w.
Fan W, Wang K, Ghasemi Falavarjani K, Sagong M, Uji A, Ip M, et al. Distribution of nonperfusion area on ultra-widefield fluorescein angiography in eyes with diabetic macular edema: DAVE study. Am J Ophthalmol. 2017;180:110–6. https://doi.org/10.1016/j.ajo.2017.05.024.
Wykoff CC, Nittala MG, Zhou B, Fan W, Velaga SB, Lampen SIR., et al. Intravitreal aflibercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3:1076–86. https://doi.org/10.1016/j.oret.2019.07.011.
Fan W, Uji A, Wang K, Falavarjani KG, Wykoff CC, Brown DM, et al. Severity of diabetic macular edema correlates with retinal vascular bed area on ultra-wide field fluorescein angiography: dave study. Retina. 2020;40:1029–37. https://doi.org/10.1097/IAE.0000000000002579.
Niki T, Muraoka K, Shimizu K. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology. 1984;91:1431–9. https://doi.org/10.1016/S0161-6420(84)34126-4.
Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–12. https://doi.org/10.1016/S0161-6420(81)34983-5.
Fang M, Fan W, Shi Y, Ip MS, Wykoff CC, Wang K, et al. Classification of regions of nonperfusion on ultra-widefield fluorescein angiography in patients with diabetic macular edema. Am J Ophthalmol. 2019;206:74–81. https://doi.org/10.1016/j.ajo.2019.03.030.
Ishibazawa A, De Pretto LR, Alibhai AY, Moult EM, Arya M, Sorour O, et al. Retinal nonperfusion relationship to arteries or veins observed on widefield optical coherence tomography angiography in diabetic retinopathy. Investig Ophthalmol Vis Sci. 2019;60:4310. https://doi.org/10.1167/iovs.19-26653.
Tsujikawa A, Ogura Y. Evaluation of leukocyte-endothelial interactions in retinal diseases. Ophthalmologica. 2012;227:68–79. https://doi.org/10.1159/000332080.
Abraham JR, Wykoff CC, Arepalli S, Lunasco L, Yu HJ, Martin A, et al. Exploring the angiographic-biologic phenotype in the IMAGINE study: quantitative UWFA and cytokine expression. Br J Ophthalmol. 2021:bjophthalmol-2020-318726. https://doi.org/10.1136/bjophthalmol-2020-318726. [Epub ahead of print.].
Couturier A, Mané V, Bonnin S, Erginay A, Massin P, Gaudric A, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015;35:2384–91. https://doi.org/10.1097/IAE.0000000000000859.
Couturier A, Rey P-A, Erginay A, Lavia C, Bonnin S, Dupas B, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126:1685–94. https://doi.org/10.1016/j.ophtha.2019.06.022.
Mané V, Dupas B, Gaudric A, Bonnin S, Pedinielli A, Bousquet E, et al. Correlation Between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina. 2016;36:S102–10. https://doi.org/10.1097/IAE.0000000000001289.
Ra H, Park JH, Baek JU, Baek J. Relationships among retinal nonperfusion, neovascularization, and vascular endothelial growth factor levels in quiescent proliferative diabetic retinopathy. JCM. 2020;9:1462. https://doi.org/10.3390/jcm9051462.
Silva PS, Cavallerano JD, Haddad NMN, Kwak H, Dyer KH, Omar AF, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949–56. https://doi.org/10.1016/j.ophtha.2015.01.008.
Fan W, Nittala MG, Fleming A, Robertson G, Uji A, Wykoff CC, et al. Relationship between retinal fractal dimension and nonperfusion in diabetic retinopathy on ultrawide-field fluorescein angiography. Am J Ophthalmol. 2020;209:99–106. https://doi.org/10.1016/j.ajo.2019.08.015.
O’Connell M, Sevgi DD, Srivastava SK, Whitney J, Hach JM, Atwood R, et al. Longitudinal precision of vasculature parameter assessment on ultra-widefield fluorescein angiography using a deep-learning model for vascular segmentation in eyes without vascular pathology. Investig Ophthalmol Vis Sci. 2020;61:2010–2010.
Sevgi DD, Srivastava SK, Wykoff C, Scott AW, Hach J, O'Connell M, et al. Deep learning-enabled ultra-widefield retinal vessel segmentation with an automated quality-optimized angiographic phase selection tool. Eye (Lond). 2021. https://doi.org/10.1038/s41433-021-01661-4. [Epub ahead of print].
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. https://doi.org/10.1016/j.ophtha.2013.02.034.
Diabetic Retinopathy Clinical Research Network GrossJG, Glassman AR, Jampol LM, Inusah S, Aiello LP, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137. https://doi.org/10.1001/jama.2015.15217.
Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021;139:946-55. https://doi.org/10.1001/jamaophthalmol.2021.2809.
Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, et al. Effect of intravitreous anti–vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the protocol w randomized clinical trial. JAMA Ophthalmol. 2021. https://doi.org/10.1001/jamaophthalmol.2021.0606.
Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–52. https://doi.org/10.1001/archophthalmol.2012.1043.
Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122:367–74. https://doi.org/10.1016/j.ophtha.2014.08.048.
Wykoff CC, Chakravarthy U, Campochiaro PA, Bailey C, Green K, Cunha-Vaz J. Long-term effects of intravitreal 0.19 mg fluocinolone acetonide implant on progression and regression of diabetic retinopathy. Ophthalmology. 2017;124:440–9. https://doi.org/10.1016/j.ophtha.2016.11.034.
Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retin. 2018;2:997–1009. https://doi.org/10.1016/j.oret.2018.06.005.
Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–9. https://doi.org/10.1016/j.ophtha.2014.03.021.
Wykoff CC, Shah C, Dhoot D, Coleman HR, Thompson D, Du W, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology. 2019;126:1171–80. https://doi.org/10.1016/j.ophtha.2019.03.040.
Figueiredo N, Srivastava SK, Singh RP, Babiuch A, Sharma S, Rachitskaya A, et al. Longitudinal panretinal leakage and ischemic indices in retinal vascular disease after aflibercept therapy: the PERMEATE study. Ophthalmol Retina. 2020;4:154–63. https://doi.org/10.1016/j.oret.2019.09.001.
Bonnin S, Dupas B, Lavia C, Erginay A, Dhundass M, Couturier A, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change. Retinal Perfusion Retin. 2019;39:426–34. https://doi.org/10.1097/IAE.0000000000002422.
Muraoka K, Shimizu K. Intraretinal neovascularization in diabetic retinopathy. Ophthalmology. 1984;91:1440–6. https://doi.org/10.1016/s0161-6420(84)34125-2.
Takahashi K, Kishi S, Muraoka K, Shimizu K. Reperfusion of occluded capillary beds in diabetic retinopathy. Am J Ophthalmol. 1998;126:791–7. https://doi.org/10.1016/S0002-9394(98)00242-6.
Krill AE, Archer DB, Newell FW, Chishti MI. Photocoagulation in diabetic retinopathy. Am J Ophthalmol. 1971;72:299–321. https://doi.org/10.1016/0002-9394(71)91300-6.
Chui TYP, Pinhas A, Gan A, Razeen M, Shah N, Cheang E, et al. Longitudinal imaging of microvascular remodelling in proliferative diabetic retinopathy using adaptive optics scanning light ophthalmoscopy. Ophthalmic Physiol Opt. 2016;36:290–302. https://doi.org/10.1111/opo.12273.
Levin AM, Rusu I, Orlin A, Gupta MP, Coombs P, D’Amico DJ, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol. 2017;11:193–200. https://doi.org/10.2147/OPTH.S118807.
Joussen AM, Poulaki V, Mitsiades N, Cai W, Suzuma I, Pak J, et al. Suppression of Fas‐FasL‐induced endothelial cell apoptosis prevents diabetic blood‐retinal barrier breakdown in a model of streptozotocin‐induced diabetes. FASEB J. 2003;17:76–8. https://doi.org/10.1096/fj.02-0157fje.
Liu Y, Shen J, Fortmann SD, Wang J, Vestweber D, Campochiaro PA. Reversible retinal vessel closure from VEGF-induced leukocyte plugging. JCI Insight. 2017;2:e95530. https://doi.org/10.1172/jci.insight.95530.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. https://doi.org/10.1126/science.1104819.
Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CYC, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13:3942–50. https://doi.org/10.1158/1078-0432.CCR-07-0278.
Sema Therapeutics. Sema Therapeutics 2018. www.semathera.com Accessed 23 Apr 2021.
Perfuse Therapeutics. Perfuse Therapeutics 2020. http://perfusetherapeutics.com/ Accessed 23 Apr 2021.
Alto LT, Terman JR. Semaphorins and their signaling mechanisms. In: Terman JR, editor. Semaphorin signaling, vol. 1493, New York, NY: Springer New York; 2017, p. 1–25. https://doi.org/10.1007/978-1-4939-6448-2_1.
Kwon SH, Shin JP, Kim IT, Park DH. Association of plasma semaphorin 3A with phenotypes of diabetic retinopathy and nephropathy. Investig Ophthalmol Vis Sci. 2016;57:2983 https://doi.org/10.1167/iovs.16-19468.
Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013;18:505–18. https://doi.org/10.1016/j.cmet.2013.09.003.
Joyal J-S, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–35. https://doi.org/10.1182/blood-2010-10-311589.
De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. https://doi.org/10.1006/exnr.2002.7884.
Fujita H, Zhang B, Sato K, Tanaka J, Sakanaka M. Expressions of neuropilin-1, neuropilin-2 and semaphorin 3A mRNA in the rat brain after middle cerebral artery occlusion. Brain Res. 2001;914:1–14. https://doi.org/10.1016/S0006-8993(01)02765-2.
Siemerink MJ, Klaassen I, Van Noorden CJF, Schlingemann RO. Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy. J Histochem Cytochem. 2013;61:101–15. https://doi.org/10.1369/0022155412467635.
Duh EJ. Sema 3A resists retinal revascularization. Blood. 2011;117:5785–6. https://doi.org/10.1182/blood-2011-03-343228.
SemaThera Board Names Garth Cumberlidge as President & CEO. BusinessWire 2018. https://www.businesswire.com/news/home/20181101005332/en/SemaThera-Board-Names-Garth-Cumberlidge-as-President-CEO. Accessed 23 Apr 2021.
Author information
Authors and Affiliations
Contributions
All authors had significant contributions to the drafting, critical revision, and supervision of the writing of the current manuscript. C.C.W. confirms final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors report the following conflicts of interest: C.C.W: consultant: Adverum, Aerie Pharmaceuticals, Allergan, Apellis, Arctic Vision, Arrowhead Pharmaceuticals, Bausch + Lomb, Bayer, Bionic Vision Technologies, Chengdu Kanghong Biotechnologies, Clearside Biomedical, EyePoint Pharmaceuticals, Genentech, Gyroscope, IVERIC Bio, Kato Pharmaceuticals, Kodiak Sciences, Long Bridge Medical, NGM Biopharmaceuticals, Novartis, OccuRx, Ocular Therapeutix, ONL Therapeutics, Opthea Limited, Oxurion, Palatin, PolyPhotonix, RecensMedical, Regeneron, RegenXBio, Roche, SAI MedPartners, Takeda, Verana Health; research support: Adverum, Aerie Pharmaceuticals, Aldeyra, Alimera Sciences, Allergan, Amgen, Apellis, Asclepix, Bayer, Boehringer Ingelheim, Chengdu Kanghong Biotechnology, Clearside Biomedical, Gemini, Genentech, Graybug Vision, Gyroscope, IONIS Pharmaceutical, iRENIX, IVERIC bio, Kodiak Sciences, LMRI, Neurotech Pharmaceuticals, NGM Biopharmaceuticals, Novartis, Oxurion, RecensMedical, Regeneron, RegenXBio, Roche, SamChunDang Pharm, Taiwan Liposome Company, Xbrane BioPharma; ownership/stock: ONL Therapeutics, PolyPhotonix, RecensMedical, Visgenx. H.J.Y: No conflicts of interest. R.L.A: consultant: Allergan, Alimera, Amgen, Bausch & Lomb, Ocular Therapeutix, Iridex, Novartis, Regeneron, RegenXbio, Santen, Genentech, and Eyepoint; stock: Novartis, Regeneron, Replenish. J.P.E: Consultant: Adverum, Aerpio, Alcon, Allegro, Allergan, Genentech/Roche, Leica, Novartis, Regeneron, Santen, Stealth, Thrombogenics, Zeiss; research support: Aerpio, Alcon, Allergan, Novartis, Regeneron, Thrombogenics; Patents: Leica. R.T: Consultant: Allergan, Alcon, Apellis, Bayer, Genentech, Iveric Bio, Chengdu Kanghong Biotechnologies, Novartis, Oculis, Roche, Thea, Zeiss; Research Support: Allergan, Bayer, Genentech, Chengdu Kanghong Biotechnologies, Novartis, Oculis, Roche. S.R.S: Consultant: Amgen, Allergan, Apellis, Genentech/Roche, Oxurion, Novartis, Regeneron, Bayer, 4DMT, Centervue, Heidelberg, Optos; research support: Carl Zeiss Meditec, Heidelberg Engineering, Optos; research instruments: Nidek, Topcon, Heidelberg, Carl Zeiss Meditec, Optos, Centervue.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wykoff, C.C., Yu, H.J., Avery, R.L. et al. Retinal non-perfusion in diabetic retinopathy. Eye 36, 249–256 (2022). https://doi.org/10.1038/s41433-021-01649-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01649-0
This article is cited by
-
CRIMSON
Die Ophthalmologie (2026)
-
Tear lactate improves the evaluation of proliferative diabetic retinopathy in type-2 diabetes patients
Molecular Biomedicine (2025)
-
New targets in diabetic retinopathy: addressing limitations of current treatments through the Sema3A/Nrp1 pathway
Eye (2025)
-
Wide-field OCTA quantified peripheral nonperfusion areas predict the risk of subclinical neovascularization
Eye (2025)
-
Diabetic retinopathy—recommendations for screening and treatment
Wiener Medizinische Wochenschrift (2025)