Abstract
Purpose
To determine the sensitivity and specificity of syphilis antibody tests in vitreous samples and to propose an algorithm using vitreous syphilis antibody as a supplementary test to confirm syphilitic uveitis (SU).
Methods
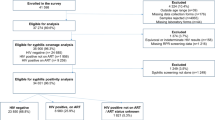
A prospective case-control study was conducted at the Retina and Uveitis Clinic from May 2017 to January 2020. Initially, patients were classified based on syphilis serology into group 1 (positive testing) and group 2 (negative testing). Group 1 was further divided into 2 subgroups (group 1A and 1B) depending on their relevant clinical manifestations and clinical improvement. Group 2 served as a control group.
Results
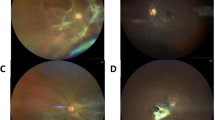
Thirty-eight patients were enrolled in the study: 14 in group 1A, 5 in group 1B, and 19 in group 2B. No patient was assigned to group 2A. All patients in group 1A, representing definite SU, completed syphilis test (rapid plasma reagin [RPR], enzyme immunoassay [EIA], and fluorescent treponemal antibody-absorption [FTA-ABS]) for vitreous, and all vitreous samples yielded positive results. Of the 5 subjects in group 1B, 3 cases were considered to be not SU with different conditions, and 2 were indeterminate for SU. They presented with different features not typical of SU, and they had variable and fewer positive syphilis antibody responses. The most sensitive test for detecting syphilis antibodies in vitreous was EIA (90.9%), followed by RPR (80.0%) and FTA-ABS IgG (78.9%). EIA and FTA-ABS had the highest specificity, detecting 100% of the syphilis antibody.
Conclusions
Vitreous analysis of syphilis antibody can serve as a supplementary test to confirm SU in selected cases as the proposed algorithm.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tuddenham S, Obeng C, Ghanem KG. Neurosyphilis and ophthalmic syphilis in persons with negative rapid plasma reagin and positive treponemal antibody test results. Sex Transm Dis. 2015;42:347–9.
Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25:513–8.
Bollemeijer JG, Wieringa WG, Missotten TO, Meenken I, ten Dam-van NH, Rothova A. et al. Clinical manifestations and outcome of syphilitic uveitis. Investig Ophthalmol Vis Sci. 2016;57:404–11.
Pichi F, Ciardella AP, Cunningham ET Jr, Morara M, Veronese C, Jumper JM, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina 2014;34:373–84.
Moradi A, Salek S, Daniel E, Gangaputra S, Ostheimer TA, Burkholder BM, et al. Clinical features and incidence rates of ocular complications in patients with ocular syphilis. Am J Ophthalmol. 2015;159:334–43 e1.
Tran TH, Cassoux N, Bodaghi B, Fardeau C, Caumes E, Lehoang P. Syphilitic uveitis in patients infected with human immunodeficiency virus. Graefes Arch Clin Exp Ophthalmol. 2005;243:863–9.
Katz AR, Komeya AY, Tomas JE. False-negative syphilis treponemal enzyme immunoassay results in an HIV-infected case-patient. Int J STD AIDS. 2017;28:735–7.
Blum L, Bachmeyer C, Caumes E. Seronegative secondary syphilis in an HIV-infected patient. Clin Exp Dermatol. 2005;30:158–9.
Ortega KL, Rezende NP, Magalhaes MH. Diagnosing secondary syphilis in a patient with HIV. Br J Oral Maxillofac Surg. 2009;47:169–70.
Geusau A, Kittler H, Hein U, Dangl-Erlach E, Stingl G, Tschachler E. Biological false-positive tests comprise a high proportion of Venereal Disease Research Laboratory reactions in an analysis of 300,000 sera. Int J STD AIDS. 2005;16:722–6.
Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4:456–66.
Smith JL, Singer JA, Moore MB Jr, Yobs AR. Seronegative ocular and neurosyphilis. Am J Ophthalmol. 1965;59:753–62.
Tamesis RR, Foster CS. Ocular syphilis. Ophthalmology 1990;97:1281–7.
Silpa-Archa S, Preble JM, Foster CS. Vitreous treponemal antibody as a supplementary test to serology for the confirmation of syphilitic chorioretinitis. Retin Cases Brief Rep. 2020;14:166–9.
Troutbeck R, Chhabra R, Jones NP. Polymerase chain reaction testing of vitreous in atypical ocular syphilis. Ocul Immunol Inflamm. 2013;21:227–30.
Ormaechea MS, Hassan M, Nguyen QD, Schlaen A. Acute syphilitic posterior placoid chorioretinopathy: an infectious or autoimmune disease? Am J Ophthalmol Case Rep. 2019;14:70–3.
Amaratunge BC, Camuglia JE, Hall AJ. Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency virus-positive and negative patients. Clin Exp Ophthalmol. 2010;38:68–74.
Rajan MS, Pantelidis P, Tong CY, French GL, Graham EM, Stanford MR. Diagnosis of Treponema pallidum in vitreous samples using real time polymerase chain reaction. Br J Ophthalmol. 2006;90:647–8.
Booth J, Rodger A, Singh J, Alexander S, Hopkins S. Syphilitic panuveitis with retinal necrosis in an HIV positive man confirmed by Treponema pallidum PCR. J Infect. 2009;59:373–5.
Grange PA, Gressier L, Dion PL, Farhi D, Benhaddou N, Gerhardt P, et al. Evaluation of a PCR test for detection of treponema pallidum in swabs and blood. J Clin Microbiol. 2012;50:546–52.
Wang C, Cheng Y, Liu B, Wang Y, Gong W, Qian Y, et al. Sensitive detection of Treponema pallidum DNA from the whole blood of patients with syphilis by the nested PCR assay. Emerg Microbes Infect. 2018;7:83.
Hoogewoud F, Frumholtz L, Loubet P, Charlier C, Blanche P, Lebeaux D, et al. Prognostic factors in syphilitic uveitis. Ophthalmology. 2017;124:1808–16.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Buitrago-Garcia D, Marti-Carvajal AJ, Jimenez A, Conterno LO, Pardo R. Antibiotic therapy for adults with neurosyphilis. Cochrane Database Syst Rev. 2019;5:CD011399.
Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. RR-03
Eandi CM, Neri P, Adelman RA, Yannuzzi LA, Cunningham ET Jr, International Syphilis Study G. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. 2012;32:1915–41.
Mathew RG, Goh BT, Westcott MC. British Ocular Syphilis Study (BOSS): 2-year national surveillance study of intraocular inflammation secondary to ocular syphilis. Investig Ophthalmol Vis Sci. 2014;55:5394–400.
Zhu J, Jiang Y, Shi Y, Zheng B, Xu Z, Jia W. Clinical manifestations and treatment outcomes of syphilitic uveitis in HIV-negative patients in China: a retrospective case study. Medicine. 2017;96:e8376.
Acknowledgements
The authors would like to thank Dr. Mongkol Tadarati and Dr. Jirawut Limwattanayingyong for referring the study participants, Dr. Jaruda Kobkitjaroen for the analysis of syphilitic testing, Mrs. Chananuch Plang-ngern, together with her colleagues, for their laboratory data support, and Mr. John Flanagan for English editing.
Author information
Authors and Affiliations
Contributions
SS conceived the study, participated in design and coordination, performed data interpretation, and drafted and edited the article. TH participated in coordination and data collection. CSF edited the article. All authors read and approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silpa-archa, S., Hoopholerb, T. & Foster, C.S. Appraisal of vitreous syphilis antibody as a novel biomarker for the diagnosis of syphilitic uveitis: a prospective case-control study. Eye 37, 146–154 (2023). https://doi.org/10.1038/s41433-021-01902-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01902-6