Abstract
Background
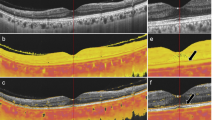
The external limiting membrane (ELM) is formed by the apical processes of Müller cells attached to the inner segments of the photoreceptor cells. Both cells are implicated in the pathogenesis of choroideremia (CHM). The purpose of this study was to explore the diagnostic role of ELM in CHM.
Methods
The study was designed as observational case series. Sixteen CHM eyes were examined by multimodal imaging and were compared to healthy controls. The main outcome was the measurement of ELM thickness and reflectivity over the follow-up, and its relationship with other multimodal imaging quantitative parameters.
Results
Baseline ELM was characterized by 11 ± 1 µm of thickness and 0.68 ± 0.13 of reflectivity, resulting 8 ± 1 µm (p < 0.01) and 0.65 ± 0.14 (p > 0.05) at the last follow-up. Choriocapillaris (CC) analysis revealed 3 regions. The first was characterized by normal vessel density (VD). The second surrounding the partially preserved islet, showing significantly lower baseline VD and undergoing minor changes over the follow-up. The third was localized in the partially preserved islet, showing significantly lower VD at baseline, and resulted atrophic at the last follow-up. ELM reflectivity and ELM thickness correlated both with outer retinal atrophy progression and the CC status.
Conclusions
ELM may be considered a useful imaging biomarker in CHM. Its assessment confirmed a primary role of Müller cells impairment in the pathogenesis of CHM.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
MacDonald IM, Sereda C, McTaggart K, Mah D. Choroideremia gene testing. Expert Rev Mol Diagn. 2004;4:478–84.
Karna J. Choroideremia. A clinical and genetic study of 84 Finnish patients and 126 female carriers. Acta Ophthalmol Suppl. 1986;176:1–68.
Roberts MF, Fishman GA, Roberts DK, Heckenlively JR, Weleber RG, Anderson RJ, et al. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol. 2002;86:658–62.
Chan SC, Bubela T, Dimopoulos IS, Freund PR, Varkouhi AK, MacDonald IM. Choroideremia research: Report and perspectives on the second international scientific symposium for choroideremia. Ophthalmic Genet. 2016;6810:1–9.
Zinkernagel MS, MacLaren RE. Recent advances and future prospects in choroideremia. Clin Ophthalmol. 2015;9:2195–200.
Jacobson SG, Cideciyan AV, Sumaroka A, Aleman TS, Schwartz SB, Windsor EAM, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Investig Ophthalmol Vis Sci. 2006;47:4113–20.
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov S, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424.
Omri S, Omri B, Savoldelli M, Jonet L, Thillaye-Goldenberg B, Thuret G, et al. The outer limiting membrane (OLM) revisited: clinical implications. Clin Ophthalmol. 2010;4:183–95.
Battaglia Parodi M, Arrigo A, MacLaren RE, Aragona E, Toto L, Mastropasqua R, et al. Vascular alterations revealed with optical coherence tomography angiography in patients with choroideremia. Retina. 2019;39:1200–5.
Arrigo A, Romano F, Parodi MB, Charbel Issa P, Birtel J, Bandello F, et al. Reduced vessel density in deep capillary plexus correlates with retinal layer thickness in choroideremia. Br J Ophthalmol. 2020: bjophthalmol-2020-316528.
Romano F, Arrigo A, MacLaren RE, Charbel Issa P, Birtel J, Bandello F, et al. Hyperreflective foci as a pathogenetic biomarker in choroideremia. Retina. 2020;40:1634–40.
Birtel J, Salvetti AP, Jolly JK, Xue K, Gliem M, Muller PL, et al. Near-infrared autofluorescence in choroideremia: anatomic and functional correlations. Am J Ophthalmol. 2019;199:19–27.
Reichenbach A, Bringmann A. Glia of the human retina. Glia. 2020;68:768–96.
van Schuppen SM, Talib M, Bergen AA, ten Brink JB, Florijn RJ, Boon CJF, et al. Long-term follow-up of patients with choroideremia with scleral pits and tunnels as a novel observation. Retina. 2018;38:1713–24.
Zweifel SA. Outer retinal tubulation: a novel optical coherence tomography finding. Arch Ophthalmol. 2009;127:1596–602.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Author information
Authors and Affiliations
Contributions
AA, MBP: study design, data analysis, data interpretation, manuscript drafting. EA, AP, FC: data collection, data analysis, manuscript revision. FB, REM: study supervision, data interpretation, manuscript revision.
Corresponding author
Ethics declarations
Competing interests
FB consultant for Alcon (Fort Worth, TX, USA), Alimera Sciences (Alpharetta, GA, USA), Allergan Inc (Irvine, CA, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, NY, USA), Genentech (San Francisco, CA, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). RM is a consultant to Biogen (Boston, USA). All other authors have no disclosures to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arrigo, A., Battaglia Parodi, M., Aragona, E. et al. Outer retinal and choriocapillaris modifications in choroideremia: three differentially impaired retinal regions and the potential diagnostic role of the external limiting membrane. Eye 37, 338–343 (2023). https://doi.org/10.1038/s41433-022-01953-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-022-01953-3