Abstract
Background
To investigate the morphological retinal parameters associated with retinal sensitivity status in retinitis pigmentosa (RP) through a quantitative multimodal imaging approach.
Methods
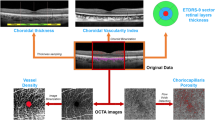
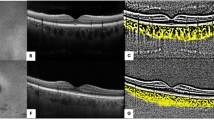
The study was designed as an observational, prospective case series, including RP patients and healthy controls. Multimodal imaging included fundus autofluorescence (FAF), structural optical coherence tomography (OCT), OCT angiography (OCTA) and microperimetry (MP). The follow-up lasted 12 months. For each imaging modality, we performed an overall quantitative analysis and a detailed investigation based on the ETDRS-9 sectors grid. Quantitative parameters included the thickness of each retinal and choroidal layer, vessel density (VD), choriocapillaris porosity (CCP), FAF intensity and MP retinal sensitivity.
Results
We included 40 eyes (40 patients) affected by RP and 40 healthy eyes (40 controls). Mean baseline BCVA was 0.14 ± 0.18 LogMAR, with 0.18 ± 0.24 LogMAR after 1-year of follow-up. RP eyes showed statistically significant alterations of retinal and choroidal layers on the ETDRS-9 sectors grid, significant reduction of VD values and MP retinal sensitivity, and significantly higher CCP than controls. The inner retinal layers proved closely associated with the functional integrity of the posterior pole. In addition, our ROC analysis provided quantitative cutoffs connected significantly with a high probability of observing a partial sparing of MP retinal sensitivity.
Conclusions
The inner retinal layers are closely associated with the functional integrity of the posterior pole in RP. FAF intensity reduction may be interpreted as lipofuscin metabolism impairment inducing increased phototoxic distress for retinal structures. Vascular involvement contributes to the morpho-functional deterioration of the macular region in RP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data may be available after formal request to the corresponding author.
References
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809.
Campochiaro PA, Mir TA. The mechanism of cone cell death in retinitis pigmentosa. Prog Retina Eye Res. 2018;62:24–37.
Iftikhar M, Kherani S, Kaur R, Lemus M, Nefalar A, Usmani B, et al. Progression of retinitis pigmentosa as measured on microperimetry: the PREP-1 study. Ophthalmol Retina. 2018;2:502–7.
Buckley TMW, Jolly JK, Josan AS, Wood LJ, Cehajic-Kapetanovic J, MacLaren RE. Clinical applications of microperimetry in RPGR-related retinitis pigmentosa: a review. Acta Ophthalmol. 2021;99:819–25.
Iacono P, Parodi MB, La Spina C, Zerbini G, Bandello F. Dynamic and static vessel analysis in patients with retinitis pigmentosa: a pilot study of vascular diameters and functionality. Retina. 2017;37:998–1002.
Arrigo A, Romano F, Albertini G, Aragona E, Bandello F, Battaglia Parodi M. Vascular patterns in retinitis pigmentosa on swept-source optical coherence tomography angiography. J Clin Med. 2019;8:1425.
Arrigo A, Bordato A, Romano F, Aragona E, Grazioli A, Bandello F, et al. Choroidal patterns in retinitis pigmentosa: correlation with visual acuity and disease progression. Transl Vis Sci Technol. 2020;9:17.
Battaglia Parodi M, La Spina C, Triolo G, Riccieri F, Pierro L, Gagliardi M, et al. Correlation of SD-OCT findings and visual function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2016;254:1275–9.
Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–65.
Funatsu J, Murakami Y, Nakatake S, Akiyama M, Fujiwara K, Shimokawa S, et al. Direct comparison of retinal structure and function in retinitis pigmentosa by co-registering microperimetry and optical coherence tomography. PLoS ONE. 2019;14:e0226097.
Jasleen KJ, Moreno M, Piers AJ, Thomas MWB, Holly B, Maclaren RE, et al. Inner retinal thickening affects microperimetry thresholds in the presence of photoreceptor thinning in patients with RPGR retinitis pigmentosa. Br J Ophthalmol. 2020:bjophthalmol-2020-317692.
Hara A, Nakazawa M, Saito M, Suzuki Y, Lewin AS. The qualitative assessment of optical coherence tomography and the central retinal sensitivity in patients with retinitis pigmentosa. PLoS ONE. 2020;15:e0232700.
Schuerch K, Woods RL, Lee W, Duncker T, Delori FC, Allikmets R, et al. Quantifying fundus autofluorescence in patients with retinitis pigmentosa. Investig Ophthalmol Vis Sci. 2017;58:1843–55.
Dysli C, Schuerch K, Escher P, Wolf S, Zinkernagel MS. Fundus autofluorescence lifetime patterns in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2018;59:1769–78.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Arrigo A, Aragona E, Saladino A, Amato A, Bandello F, Battaglia Parodi M. The impact of different thresholds on optical coherence tomography angiography images binarization and quantitative metrics. Sci Rep. 2021;11:14758.
Parodi B, Nieto A, Albrecht V, Maas J, Orth M, Neumaier K, et al. Multimodal imaging in subclinical best vitelliform macular dystrophy. Br J Ophthalmol. 2020;15:19. bjophthalmol-2020-317635.
Parodi MB, Triolo G, Morales M, Borrelli E, Cicinelli MV, Cascavilla ML, et al. MP1 and MAIA fundus perimetry in healthy subjects and patients affected by retinal dystrophies. Retina. 2015;35:1662–9.
Battaglia Parodi M, Castellino N, Iacono P, Chowers I, Empeslidis T, Goldstein M, et al. Microperimetry in Best vitelliform macular dystrophy. Retina. 2018;38:841–8.
Kim JH, Lee HS, Kim NR, Seong GJ, Kim CY. Relationship between visual acuity and retinal structures measured by spectral domain optical coherence tomography in patients with open-angle glaucoma. Investig Ophthalmol Vis Sci. 2014;55:4801–11.
Rebolleda G, Sánchez-Sánchez C, González-López JJ, Contreras I, Muñoz-Negrete FJ. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015;56:682–92.
Vamos R, Tatrai E, Nemeth J, Holder GE, DeBuc DC, Somfai GM. The structure and function of the macula in patients with advanced retinitis pigmentosa. Investig Ophthalmol Vis Sci. 2011;52:8425–32.
Liu G, Liu X, Li H, Du Q, Wang F. Optical coherence tomographic analysis of retina in retinitis pigmentosa patients. Ophthalmic Res. 2016;56:111–22.
Nagasaka Y, Ito Y, Ueno S, et al. Inner retinal layer thickness in eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014;55:1725.
Huang WC, Cideciyan AV, Roman AJ, Sumaroka A, Sheplock R, Schwartz SB, et al. Inner and outer retinal changes in retinal degenerations associated with ABCA4 mutations. Investig Ophthalmol Vis Sci. 2014;55:1810–22.
Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–9.
Lee J, Asano S, Inoue T, Fujino Y, Matsuura M, Kitamoto K, et al. Investigating the usefulness of fundus autofluorescence in retinitis pigmentosa. Ophthalmol Retin. 2018;2:1062–70.
Kellner U, Kellner S, Weber BH, Fiebig B, Weinitz S, Ruether K. Lipofuscin- and melanin-related fundus autofluorescence visualize different retinal pigment epithelial alterations in patients with retinitis pigmentosa. Eye. 2009;23:1349–59.
Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci. 2014;34:11504–13.
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106:18751–6.
Saint-Geniez M, Maharaj ASR, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, et al. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS ONE. 2008;3:e3554.
Froger N, Matonti F, Roubeix C, Forster V, Ivkovic I, Brunel N, et al. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci Rep. 2020;10:12409.
Hu J, Zhu M, Li D, Wu Q, Le YZ. VEGF as a direct functional regulator of photoreceptors and contributing factor to diabetes-induced alteration of photoreceptor function. Biomolecules. 2021;11:988.
Tani T, Nagaoka T, Nakabayashi S, Yoshioka T, Yoshida A. Autoregulation of retinal blood flow in response to decreased ocular perfusion pressure in cats: comparison of the effects of increased intraocular pressure and systemic hypotension. Investig Ophthalmol Vis Sci. 2014;55:360–7.
Weerasekera LY, Balmer LA, Ram R, Morahan G. Characterization of retinal vascular and neural damage in a novel model of diabetic retinopathy. Investig Ophthalmol Vis Sci. 2015;56:3721–30.
Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140195.
Funding
FB consultant for: Alcon (Fort Worth, TX, USA), Alimera Sciences (Alpharetta, GA, USA), Allergan Inc (Irvine, CA, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, NY, USA), Genentech (San Francisco, CA, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). All other authors have no disclosures to declare.
Author information
Authors and Affiliations
Contributions
AA, EA: study design, study conduction, data analysis, data interpretation, manuscript draft. CP, AS, AA, LB, AP, GB: data acquisition, data analysis, revision of the literature, critical revision of the manuscript. FB, MBP: data interpretation, critical revision of the manuscript, supervision of the entire study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arrigo, A., Aragona, E., Perra, C. et al. Morphological and functional involvement of the inner retina in retinitis pigmentosa. Eye 37, 1424–1431 (2023). https://doi.org/10.1038/s41433-022-02139-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-022-02139-7