Abstract
Objectives
To compare visual outcomes for low vision eyes (LVE) (<35 letters LogMAR or <20/200 Snellen) versus non-low vision eyes (NLVE) (>35 letters LogMAR or >20/200 Snellen) at the time of the first injection in a clinical practice setting.
Methods
Subgroup analysis of a multicenter national database of treatment- naïve eyes neovascular age related macular degeneration (nAMD) treated with anti-VEGF intravitreal injections divided into LVE and NLVE. Demographics, visual acuity (VA) at baseline and subsequent timepoints (12, 24, and 36 months), number of injections and visits data were collected using a validated web-based tool (Fight Retinal Blindness!).
Results
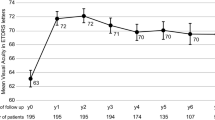
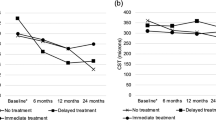
3138 eyes were included, 705 LVE and 2433 NLVE. The LVE group had the greatest VA gain (p < 0.001), at 12, 24, and 36 months (+15, +15, and +13 letters respectively). The proportion of patients with VA loss (−5 letters) differed between groups at 12, 24, and 36 and was significantly greater (p < 0.001) in NLVE. The proportion of patients with VA gain (+5 letters) was significantly higher (p < 0.001) in LVE in all timeframes. The proportions of LVE that still had VA ≤ 35 letters at 12, 24, and 36 months were 54%, 54%, and 57%. Conversely, 8%, 9%, and 8% of LVE achieved VA ≥ 70 letters at 12, 24, and 36 months. LVE received fewer intravitreal injections than NLVE throughout follow-up (6, 9, 12 vs 7, 11, 15).
Conclusion
Findings of this study support the need for ongoing therapy in patients with initial visual acuity less than 35 letters since sustained visual improvements can be achieved and maintained for the first 3 years of treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. All data supporting the findings of this study are included in this published article (and its Supplementary Information files).
References
Heier JS, Brown DM, Chong V, Korobelnik J, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. https://doi.org/10.1016/j.ophtha.2012.09.006.
Martin DF, Ying G, Grunwald JE, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. https://doi.org/10.1056/NEJMOA1102673/SUPPL_FILE/NEJMOA1102673_DISCLOSURES.PDF.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. https://doi.org/10.1056/NEJMoa054481.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. https://doi.org/10.1056/NEJMoa062655.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. https://doi.org/10.1016/j.ophtha.2012.03.053.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411. https://doi.org/10.1016/j.ophtha.2012.04.015.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–67. https://doi.org/10.1016/S0140-6736(13)61501-9.
Ho AC, Busbee BG, Regillo CD, Heier JS, Lindstrom RL, Orr SC, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121:2181–92. https://doi.org/10.1016/J.OPHTHA.2014.05.009.
Busbee BG, Ho AC, Brown DM, Heier JS, Suñer IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56. https://doi.org/10.1016/J.OPHTHA.2012.10.014.
Bressler NM, Doan QV, Varma R, Lee PP, Suñer IJ, Dolan C, et al. Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularization: non-Hispanic white population in the United States with age-related macular degeneration. Arch Ophthalmol (Chic, Ill 1960). 2011;129:709–17. https://doi.org/10.1001/ARCHOPHTHALMOL.2011.140.
Zarranz-Ventura J, Parrado-Carrillo A, Nguyen V, Sararols L, Garay-Aramburu G, Puzo M, et al. Creation of a neovascular age-related macular degeneration national database using a web-based platform: Fight Retinal Blindness Spain. Report 1: Visual outcomes. Clin Exp Ophthalmol. 2022;50:312–24. https://doi.org/10.1111/CEO.14054.
Ozawa Y, Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, et al. Clinical medicine managing neovascular age-related macular degeneration in clinical practice: systematic review, meta-analysis, and meta-regression. J Clin Med. 2022. https://doi.org/10.3390/jcm11020325.
Martin-Pinardel R, Izquierdo-Serra J, De Zanet S, Parrado-Carrillo A, Garay-Aramburu G, Puzo M, et al. Artificial intelligence-based fluid quantification and associated visual outcomes in a real-world, multicentre neovascular age-related macular degeneration national database. Br J Ophthalmol. 2024;108:253–62. https://doi.org/10.1136/bjo-2022-322297.
Recommendations | Age-related macular degeneration | Guidance | NICE.
Yeung L, Hsieh YT, Yang CH, Chen LJ, Chen SJ, Cheng CK, et al. Management of neovascular age-related macular degeneration: Taiwan expert consensus. J Formos Med Assoc. 2021;120:2061–71. https://doi.org/10.1016/J.JFMA.2021.06.012.
Gillies MC, Walton R, Liong J, Arnold JJ, McAllister I, Moriet N, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! project. Retina. 2014;34:188–95. https://doi.org/10.1097/IAE.0b013e318296b271.
Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–42. https://doi.org/10.1007/s00417-008-0926-0.
Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Exp Ophthalmol. 2011;39:5–8. https://doi.org/10.1111/J.1442-9071.2010.02424.X.
Rahimy E, Rayess N, Ho AC, Regillo CD. Treatment outcomes for neovascular age-related macular degeneration patients with initial vision better than 20/40 using a treat-and-extend regimen. Retina. 2016;36:875–80. https://doi.org/10.1097/IAE.0000000000000814.
Langelaan M, De Boer MR, Van Nispen RMA, Wouters B, Moll AC, Van Rens GHMB. Impact of visual impairment on quality of life: a comparison with quality of life in the general population and with other chronic conditions. Ophthalmic Epidemiol. 2007;14:119–26. https://doi.org/10.1080/09286580601139212.
Fenwick EK, Ong PG, Man REK, Cheng CY, Sabanayagam C, Wong TY, et al. Association of Vision Impairment and Major Eye Diseases With Mobility and Independence in a Chinese Population. JAMA Ophthalmol. 2016;134:1087–93. https://doi.org/10.1001/JAMAOPHTHALMOL.2016.2394.
Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, Lanzetta P. Managing neovascular age-related macular degeneration in clinical practice: systematic review, meta-analysis, and meta-regression. J Clin Med. 2022;11. https://doi.org/10.3390/JCM11020325.
Gillies MC, Campain A, Barthelmes D, Simpson JM, Arnold JJ, Guymer RH, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:1837–45. https://doi.org/10.1016/j.ophtha.2015.05.010.
Tufail A. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity manuscript no. 2013-568. Ophthalmology. 2014;121:1092–101. https://doi.org/10.1016/j.ophtha.2013.11.031.
Zarranz-Ventura J, Liew G, Johnston RL, Xing W, Akerele T, McKibbin M, et al. The neovascular age-related macular degeneration database: Report 2: Incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 2014;121:1966–75. https://doi.org/10.1016/j.ophtha.2014.04.026.
Izquierdo-Serra J, Martin-Pinardel R, Moll-Udina A, Bernal-Morales C, Garay-Aramburu G, Sanchez-Monroy J, et al. Macular neovascularization type influence on anti-VEGF intravitreal therapy outcomes in age-related macular degeneration. Ophthalmol Retin. 2024;8:350–9. https://doi.org/10.1016/j.oret.2023.10.022.
Acknowledgements
The authors would like to thank Sonia de Orte Seemann and Cristina Martinez-García from Syneos Health for their invaluable help in the overall management of this study; Sarah Steinmann for the web-based support with the FRB! platform; Guillem Masdeu, Miguel Moratalla, Judith Martinez, Daniel Garcés, Maria Jose Gonzalez and Antonia Soriano from the Fundació Recerca Clínic Barcelona—Institut de Investigacions Biomediques August Pi I Sunyer (FRCB-IDIBAPS) for the administrative support with contracts and legal issues; and Laia Gomez-Baldo and Carmen Navarro from Novartis Spain for the external support during the development of this study.
Funding
This work was supported in part by a grant from Novartis Pharmaceuticals. No member or affiliate of Novartis had any input into data analysis, interpretation of the data, or writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
MP, PCP, FBP, and JZV designed the study, conducted the research, and analyzed/interpreted data, MP wrote the manuscript, JZV is the main director and the national coordinator of the FRB! Spain project. PC, FBP, and JZV collected data and edited the manuscript. RMP performed the statistical analysis. MCG is one of the inventors of the software and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JZV, PCP, and MCG receive funding and consult for Novartis Pharmaceuticals and Bayer. JZV is a member of the Eye editorial board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puzo, M., Calvo-Perez, P., Bartol-Puyal, F. et al. Fight Retinal Blindness SPAIN. Report 3: clinical outcomes of vascular endothelial growth factor inhibitors in low vision eyes with neovascular age-related macular degeneration. A national database study. Eye 38, 3450–3458 (2024). https://doi.org/10.1038/s41433-024-03322-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-024-03322-8