Abstract
Purpose
To assess relationship between dark without pressure (DWP) retinopathy and retinal vasculitis (RV) in paediatric uveitis patients.
Methods
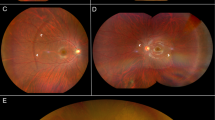
This is a retrospective cross-sectional study. Wide angle fundus photographs (WAFP), fundus autofluorescence (FAF), and optical coherence tomography (OCT) images were analysed to identify DWP retinopathy. Confluent dark areas on WAFP, hypo-autofluorescence on FAF, and EZ disruption on OCT images were the markers for DWP. DWP retinopathy area was measured using Image-J. The prevalence and characteristics of DWP in RV patients and its association with RV was reported.
Results
Out of 43 paediatric uveitis patients, 26 were diagnosed with RV. Amongst these, DWP was detected in 20 patients (30 eyes) which were analysed. Mean age was 12.8 ± 3.36 years; 40% were female. DWP areas were either diffuse, mid-peripheral or peripheral on WAFP. All 20 patients (30 eyes) showed hypo-autofluorescence on FAF at the same locations as the WAFP. The mean duration of follow-up was 27.2 ( ± 13.7) months. 12 patients (19 eyes) who had DWP at baseline visit had longer duration of uveitis compared to 8 patients (11 eyes) who developed DWP on follow-up visits (p < 0.036). Progression of the retinopathy overtime was analysed in 28 eyes; all eyes showed improvement of RV with therapy. There was a statistically significant relationship between change in vascular leakage and the change in DWP retinopathy area (chi square= 11.67, p = 0.001).
Conclusions
DWP can potentially serve as a biomarker of RV in paediatric uveitis patients and its presence should warrant WAFA evaluation in this patient population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data can be made available upon reasonable request after removing appropriate patient identifiers.
References
Abu El-Asrar AM, Herbort CP, Tabbara KF. Retinal vasculitis. Ocul Immunol Inflamm. 2005;13:415–33.
Yang P, Zhong Z, Su G, Ye X, Tan S, Li F, et al. Retinal Vasculitis, a Common Manifestation of Idiopathic Pediatric Uveitis?. Retina. 2021;41:610–9.
Agarwal A, Afridi R, Agrawal R, Do DV, Gupta V, Nguyen QD. Multimodal Imaging in Retinal Vasculitis. Ocul Immunol Inflamm. 2017;25:424–33.
El-Asrar AM, Herbort CP, Tabbara KF. A clinical approach to the diagnosis of retinal vasculitis. Int Ophthalmol. 2010;30:149–73.
Shrestha JK, Khadka D, Lamichhane G, Khanal S. Retinal vasculitis. Nepal J Ophthalmol. 2009;1:66–71.
Diala FGI, McCarthy K, Chen JL, Tsui E. Multimodal imaging in pediatric uveitis. Ther Adv Ophthalmol. 2021;13:25158414211059244.
Nagpal KC, Goldberg MF, Asdourian G, Goldbaum M, Huamonte F. Dark-without-pressure fundus lesions. Br J Ophthalmol. 1975;59:476–9.
Fawzi AA, Nielsen JS, Mateo-Montoya A, Somkijrungroj T, Li HK, Gonzales J, et al. Multimodal imaging of white and dark without pressure fundus lesions. Retina. 2014;34:2376–87.
Chang MY, McBeath JB, McCannel CA, McCannel TA. Shadow sign’ in congenital hypertrophy of the retinal pigment epithelium of young myopic pigmented patients. Eye (Lond). 2016;30:160–3.
Condon PI, Serjeant GR. Ocular findings in hemoglobin SC disease in Jamaica. Am J Ophthalmol. 1972;74:921–31.
Condon PI, Serjeant GR. Ocular findings in homozygous sickle cell anemia in Jamaica. Am J Ophthalmol. 1972;73:533–43.
Flores Pimentel MA, Duncan JL, de Alba Campomanes AG, Moore A. Dark without pressure retinal changes in a paediatric age group. Eye. 2021;35:1221–7.
Sherman T, Palileo BM, Adam CR, Abrams GW. Dark without pressure in a case of choroidal osteoma. Retin Cases Brief Rep. 2020;16:593–596.
Steptoe PJ, Momorie F, Fornah AD, Komba SP, Emsley E, Scott JT, et al. Multimodal Imaging and Spatial Analysis of Ebola Retinal Lesions in 14 Survivors of Ebola Virus Disease. JAMA Ophthalmol. 2018;136:689–93.
Steptoe PJ, Momorie F, Fornah AD, Komba P, Emsley E, Scott JT, et al. Evolving longitudinal retinal observations in a cohort of survivors of Ebola virus disease. JAMA Ophthalmol. 2020;138:395–403.
Lott PW, McKibbin M. Prevalence of dark without pressure and angioid streaks in sickle cell disease. Ophthalmic Surg Lasers Imaging Retin. 2021;52:620–2.
J Steptoe P, Guly CM, Dick AD. Ocular toxoplasmosis associated dark without pressure. Ocul Immunol Inflamm. 2022;31:624–626.
Bittencourt MG, Hassan M, Halim MS, Afridi R, Nguyen NV, Plaza C, et al. Blue light versus green light fundus autofluorescence in normal subjects and in patients with retinochoroidopathy secondary to retinal and uveitic diseases. J Ophthalmic Inflamm Infect. 2019;9:1.
Cuenca N, Ortuno-Lizaran I, Pinilla I. Cellular Characterization of OCT and Outer Retinal Bands Using Specific Immunohistochemistry Markers and Clinical Implications. Ophthalmology. 2018;125:407–22.
Litts KM, Zhang Y, Freund KB, Curcio CA. Optical Coherence Tomography and Histology of Age-Related Macular Degeneration Support Mitochondria as Reflectivity Sources. Retina. 2018;38:445–61.
Tychinsky V. The metabolic component of cellular refractivity and its importance for optical cytometry. J Biophoton. 2009;2:494–504.
Wilson JD, Bigelow CE, Calkins DJ, Foster TH. Light scattering from intact cells reports oxidative-stress-induced mitochondrial swelling. Biophys J. 2005;88:2929–38.
Mirra S, Marfany G. Mitochondrial Gymnastics in Retinal Cells: A Resilience Mechanism Against Oxidative Stress and Neurodegeneration. Adv Exp Med Biol. 2019;1185:513–7.
Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012;13:909–15.
Zheng JY, Tsai YC, Kadimcherla P, Zhang R, Shi J, Oyler GA, et al. The C-terminal transmembrane domain of Bcl-xL mediates changes in mitochondrial morphology. Biophys J. 2008;94:286–97.
Tychinsky V, Kretushev A, Vyshenskaja T. Mitochondria optical parameters are dependent on their energy state: a new electrooptical effect?. Eur Biophys J. 2004;33:700–5. Dec.
Rutter KM, Hutto RA, Brockerhoff SE. Photoreceptor mitochondria can be transferred and turned over by Müller glia. Invest Ophthalmol Visual Sci. 2022;63:2579 – F0462-2579 – F0462.
Saxena S, Meyer CH, Akduman L. External limiting membrane and ellipsoid zone structural integrity in diabetic macular edema. Eur J Ophthalmol. 2021;32:15–16.1611206721211026106.
Sinha S, Saxena S, Prasad S, Mahdi AA, Bhasker SK, Das S, et al. Association of serum levels of anti-myeloperoxidase antibody with retinal photoreceptor ellipsoid zone disruption in diabetic retinopathy. J Diabetes Complications. 2017;31:864–8.
Mori Y, Suzuma K, Uji A, Ishihara K, Yoshitake S, Fujimoto M, et al. Restoration of foveal photoreceptors after intravitreal ranibizumab injections for diabetic macular edema. Sci Rep. 2016;6:39161.
De S, Saxena S, Kaur A, Mahdi AA, Misra A, Singh M, et al. Sequential restoration of external limiting membrane and ellipsoid zone after intravitreal anti-VEGF therapy in diabetic macular oedema. Eye (Lond). 2021;35:1490–5.
Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606.
Vives-Bauza C, Anand M, Shiraz AK, Magrane J, Gao J, Vollmer-Snarr HR, et al. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–80.
Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl. 2002;41:814–7.
Eskandarpour M, Nunn MA, Weston-Davies W, Calder VL. Immune-mediated retinal vasculitis in posterior uveitis and experimental models: the leukotriene (LT)B4-VEGF Axis. Cells. 2021;10:396.
Funding
An unrestricted grant from Research to Prevent Blindness, National Eye Institute, P30-Ey026877.
Author information
Authors and Affiliations
Contributions
Çigdem Yasar: Initial observations and hypothesis development, methodology, data collection, image analysis, data analysis, interpretation, writing the original draft, reviewing, and revisions. Muhammad Hassan: Reviewing and writing the original and revised manuscript, methodology, data interpretation, and analysis. Hashem Ghoraba: Manuscript review and methodology. Christopher Or: Manuscript review and image analysis-software support. Amir Akhavanrezayat: Manuscript review and image preparation. Jonathan Regenold: Manuscript review. Sungwho Park: Manuscript review. Hassan Khojasteh: Manuscript review. Muhammad Sohail Hali: Manuscript review and image analysis. Irmak Karaca: Manuscript review. Brandon Huy Pham: Manuscript review. Gunay Uludag: Manuscript review. Wataru Matsumiya: Manuscript review. Quan Dong Nguyen: Manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yasar, C., Hassan, M., Ghoraba, H. et al. Multimodal imaging of dark without pressure lesions in paediatric retinal vasculitis: A cross-sectional study. Eye 39, 2645–2654 (2025). https://doi.org/10.1038/s41433-025-03882-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03882-3