Abstract
Objectives
To demonstrate effect of completion time of initiation phase of anti-vascular endothelial growth factor (anti-VEGF) agents on visual and anatomical outcomes in diabetic macular oedema patients.
Methods
This multicentre, retrospective, comparative study included a subgroup of patients from MARMASIA Study. Two groups were formed according to completion time of initiation phase of anti-VEGF treatment as ideal and extended completion time groups (ICT and ECT groups). Changes in best corrected visual acuity (BCVA) as ETDRS letter score, central macular thickness (CMT) were compared between the groups at 3rd, 6th, 12th months. At 12th month injection, visit numbers were also evaluated.
Results
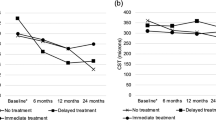
A total of 328 eyes of 239 patients were included. Ninety-five eyes were in ICT, 233 eyes were in ECT group. Mean BCVA was significantly better than the baseline at 3rd, 6th, 12th months in ICT and ECT groups (p < 0.001 for all). Mean change in BCVA at months 3, 6, 12 was significantly better in the ICT group than the ECT group (p < 0.001, p = 0.003, p = 0.03, respectively). The mean CMT from baseline to at 3rd, 6th, 12th months was significantly decreased in the ICT and ECT groups (p < 0.0001 for all). Change in mean CMT was significantly higher in the ICT group than in the ECT group at 3rd, 6th, 12th months (p < 0.0001 for all). Mean number of injections, visits were not significantly different between groups (p = 0.2).
Conclusions
Initiation phase injections must be completed within a maximum of 70 days (between injections 28 + 7 days) for better visual and anatomical outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91.
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85.
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol. 2016;172:72–9.
Glassman AR, Wells JA 3rd, Josic K, Maguire MG, Antoszyk AN, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T extension study). Ophthalmology. 2020;127:1201–10.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91.
Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retin. 2018;2:1179–87.
Van Aken E, Favreau M, Ramboer E, Denhaerynck K, MacDonald K, Abraham I, et al. Realworld outcomes in patients with diabetic macular edema treated long term with ranibizumab (VISION study). Clin Ophthalmol. 2020;14:4173–85.
Massin P, Creuzot-Garcher C, Kodjikian L, Girmens JF, Delcourt C, Fajnkuchen F, et al. Real-world outcomes after 36-month treatment with ranibizumab 0.5 mg in patients with visual impairment due to diabetic macular edema (BOREAL-DME). Ophthalmic Res. 2021;64:577–86.
Choovuthayakorn J, Phinyo P, Tantraworasin A, Kunavisarut P, Patikulsila D, Chaikitmongkol V, et al. Intravitreal anti-vascular endothelial growth factor therapy for diabetic macular edema in clinical practice of single center: three-year outcomes. Ophthalmic Res. 2021;64:483–93.
Naujokaitis T, Balciuniene VJ. Real-world treatment patterns and vision outcomes with ranibizumab for diabetic macular edema. J Ophthalmol. 2021;2021:8825082.
Peto T, Akerele T, Sagkriotis A, Zappacosta S, Clemens A, Chakravarthy U. Treatment patterns and persistence rates with anti-vascular endothelial growth factor treatment for diabetic macular oedema in the UK: A real-world study. Diabet Med. 2022;39:e14746.
Sugimoto M, Handa C, Hirano K, Sunaya T, Kondo M. Intravitreal aflibercept for diabetic macular edema in real-world clinical practice in Japan: 24-month outcomes. Graefes Arch Clin Exp Ophthalmol. 2022;260:3489–98.
Durukan AH, Unlu N, Onen M, Alp MN, Yesiltas YS, Kalayci D, et al. Anti-vascular endothelial growth factor therapy in diabetic macular edema: real-life outcomes from a multicenter study in Turkey over 36 months. Int Ophthalmol. 2022;42:3777–87.
Yuen YS, Tan GSW, Gan NY, Too IHK, Mothe RK, Basa P, et al. Real-world evidence in the management of diabetic macular edema with intravitreal anti-VEGFs in Asia: A systematic literature review. Clin Ophthalmol. 2022;16:3503–26.
Maggio E, Sartore M, Attanasio M, Maraone G, Guerriero M, Polito A, et al. Anti-vascular endothelial growth factor treatment for diabetic macular edema in a real-world clinical setting. Am J Ophthalmol. 2018;195:209–22.
Schwarzer P, Ebneter A, Munk M, Wolf S, Zinkernagel MS. One-year results of using a treat-and-extend regimen without a loading phase with anti-VEGF agents in patients with treatment-naive diabetic macular edema. Ophthalmologica. 2019;241:220–5.
James DGP, Mitkute D, Porter G, Vayalambrone D. Visual outcomes following intravitreal ranibizumab for diabetic macular edema in a pro re nata protocol from baseline: A real-world experience. Asia Pac J Ophthalmol. 2019;8:200–5.
Talks SJ, Stratton I, Peto T, Lotery A, Chakravarthy U, Eleftheriadis H, et al. Aflibercept in clinical practice; visual acuity, injection numbers and adherence to treatment, for diabetic macular oedema in 21 UK hospitals over 3 years. Eye. 2022;36:72–7.
Mitchell P, Sheidow TG, Farah ME, Mahmood S, Minnella AM, Eter N, et al. Effectiveness and safety of ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: Results from the real-world global LUMINOUS study. PLoS ONE. 2020;15:e0233595.
Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2021;105:216–21.
Creuzot Garcher C, Massin P, Kodjikian L, Girmens J-F, DelCourt C, Fajnkuchen F, et al. Real-world outcomes with ranibizumab 0.5 mg treatment in French patients with visual impairment due to diabetic macular edema: 12-month results from the 36-month BOREALDME study. Invest Ophthalmol Vis Sci. 2017;58:1915.
Fourmax E, Lecleire-Collet A, Dot C, Le Lez M-L, Baillif S, Erginay A, et al. Real-world outcomes with ranibizumab 0.5 mg in treatment-naïve French patients with visual impairment due to diabetic macular edema: 12-month results from the ETOILE study. Invest Ophthalmol Vis Sci. 2017;58:4625.
Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80.
Yayla U, Sevik MO, Karabaş VL, Şahin Ö, Özkaya A, Yenerel NM, et al. Real-World Outcomes of Intravitreal Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema in Türkiye: MARMASIA Study Group Report No. 1. Turk J Ophthalmol. 2023;53:356–68.
Beck RW, Moke PS, Turpin AH, Ferris FL 3rd, SanGiovanni JP, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205.
Pierro L, Gagliardi M, Iuliano L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012;53:5912–20.
Author information
Authors and Affiliations
Contributions
Concept and design: TAG, AÖ, LK. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: TAG, AÖ, MOS. Critical review of the manuscript for important intellectual content: All authors. Supervision: LK, ÖŞ.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there is no conflict of interests. All authors attest that they meet the current ICMJE criteria for authorship.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors certify that this work is original and has not been published elsewhere, nor is it currently under consideration for publication anywhere. None of the authors have any conflicts of interest to disclose. No funding was received for conducting this study. The authors have no financial interests to report.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydoğan Gezginaslan, T., Özkaya, A., Sevik, M.O. et al. Does completion time of the initiation phase of anti-vascular growth factor affect visual and anatomical outcomes?: The MARMASIA study group report No.5. Eye 39, 2579–2583 (2025). https://doi.org/10.1038/s41433-025-03928-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03928-6