Abstract
Objective
To compare the effectiveness of intravitreal faricimab and aflibercept injections in patients with polypoidal choroidal vasculopathy (PCV).
Methods
This retrospective study analysed 111 treatment-naïve eyes (111 patients) with PCV who received intravitreal injections of either faricimab (30 eyes) or aflibercept (81 eyes). All patients were treated with three initial monthly loading injections. Visual and anatomical outcomes were compared between the two treatment groups after 12 months.
Results
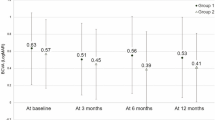
After 12 months of treatment, mean best-corrected visual acuity (BCVA) improved significantly in both the faricimab-treated and aflibercept groups (P < 0.05), with no significant difference in the extent of improvement between the two groups. Similarly, no significant differences were found in the reduction of central retinal thickness or subfoveal choroidal thickness, nor in the proportion of eyes with dry macula after treatment. However, the faricimab group showed a significantly greater reduction in maximum pigment epithelial detachment (PED) thickness from baseline than the aflibercept group (48.2% vs. 38.7%; P = 0.015). A higher proportion of eyes in the faricimab group also showed a PED thickness reduction >50% (66.7% vs. 42.0%; P = 0.021). The rate of polypoidal lesion regression did not differ significantly between the groups (50.0% vs. 38.3%; P = 0.256).
Conclusion
Intravitreal faricimab injections resulted in visual and anatomical improvements comparable to aflibercept in patients with PCV over 12 months of treatment, including a similar rate of polyp regression. In addition, faricimab was more effective in reducing PED thickness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, Freund KB, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125:708–24.
Song SJ, Youm DJ, Chang Y, Yu HG. Age-related macular degeneration in a screened South Korean population: prevalence, risk factors, and subtypes. Ophthalmic Epidemiol. 2009;16:304–10.
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Koh AH, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33:686–716.
Cho HJ, Kim KM, Kim HS, Han JI, Kim CG, Lee TG, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1–6.
Shirley M. Faricimab: first approval. Drugs. 2022;82:825–30.
Nair AA, Finn AP, Sternberg P Jr. Spotlight on faricimab in the treatment of wet age-related macular degeneration: design, development and place in therapy. Drug Des Devel Ther. 2022;16:3395–400.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40.
Mukai R, Kataoka K, Tanaka K, Miyara Y, Maruko I, Nakayama M, et al. One-year outcomes and safety assessment of faricimab in treatment-naive patients with neovascular age-related macular degeneration in Japan. Sci Rep. 2024;14:11681.
Mukai R, Kataoka K, Tanaka K, Miyara Y, Maruko I, Nakayama M, et al. Three-month outcomes of faricimab loading therapy for wet age-related macular degeneration in Japan. Sci Rep. 2023;13:8747.
Ma L, Brelen ME, Tsujikawa M, Chen H, Chu WK, Lai TY, et al. Identification of ANGPT2 as a new gene for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in the Chinese and Japanese populations. Invest Ophthalmol Vis Sci. 2017;58:1076–83.
Cho HJ, Kim KM, Kim HS, Lee DW, Kim CG, Kim JW. Response of pigment epithelial detachment to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol. 2016;166:112–9.
Sharma A, Kumar N, Parachuri N, Bandello F, Kuppermann BD, Loewenstein A. Faricimab: two in the bush is proving better than one in the hand?. Ocul Immunol Inflamm. 2022;30:1961–3.
Nicolo M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30:193–200.
Todoroki T, Takeuchi J, Ota H, Nakano Y, Sajiki AF, Nakamura K, et al. Aqueous humor cytokine analysis in age-related macular degeneration after switching from aflibercept to faricimab. Invest Ophthalmol Vis Sci. 2024;65:15.
Khanani AM, Kotecha A, Chang A, Chen SJ, Chen Y, Guymer R, et al. TENAYA and LUCERNE: two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology. 2024;131:914–26.
Khanani AM, Guymer RH, Basu K, Boston H, Heier JS, Korobelnik JF, et al. TENAYA and LUCERNE: rationale and design for the phase 3 clinical trials of faricimab for neovascular age-related macular degeneration. Ophthalmol Sci. 2021;1:100076.
Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1912–20.
Khanani AM, Aziz AA, Khan H, Gupta A, Mojumder O, Saulebayeva A, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study - 6 month results. Eye. 2023;37:3574–81.
Veritti D, Sarao V, Gonfiantini M, Rubinato L, Lanzetta P. Faricimab in neovascular AMD complicated by pigment epithelium detachment: an AI-assisted evaluation of early morphological changes. Ophthalmol Ther. 2024;13:2813–24.
Simader C, Ritter M, Bolz M, Deak GG, Mayr-Sponer U, Golbaz I, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121:1237–45.
Schmidt-Erfurth U, Waldstein SM, Deak GG, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:822–32.
Vyas CH, Cheung CMG, Jordan-Yu JMN, Shimizu H, Tan ACS, Sim SS, et al. Novel volumetric imaging biomarkers for assessing disease activity in eyes with PCV. Sci Rep. 2022;12:2993.
Tsujikawa A, Sasahara M, Otani A, Gotoh N, Kameda T, Iwama D, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007;143:102–11.
Fenner BJ, Cheung CMG, Sim SS, Lee WK, Staurenghi G, Lai TYY, et al. Evolving treatment paradigms for PCV. Eye. 2022;36:257–65.
Cho HJ, Kang KH, Yoon W, Lee J, Kim CG, Kim JW. Intravitreal brolucizumab and aflibercept for polypoidal choroidal vasculopathy. J Ocul Pharm Ther. 2023;39:653–60.
Ito A, Maruyama-Inoue M, Kitajima Y, Ikeda S, Inoue T, Kadonosono K. One-year outcomes of intravitreal brolucizumab injections in patients with polypoidal choroidal vasculopathy. Sci Rep. 2022;12:7987.
Acknowledgements
This study was supported by Kim’s Eye Hospital Research Center.
Author information
Authors and Affiliations
Contributions
Design and conduction of the study (HJC); Data collection (HJC, HYH, SP, IY, and JHK); Analysis and interpretation of data (HJC and JC); Writing of the article (HJC and JC); Critical revision and final approval of article (HJC).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, J., Han, H.Y., Park, S. et al. One-year outcomes of faricimab versus aflibercept treatment for polypoidal choroidal vasculopathy: a comparative study. Eye 39, 2893–2898 (2025). https://doi.org/10.1038/s41433-025-03980-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03980-2