Abstract
Background
Blepharitis, meibomian gland dysfunction (MGD), and chalazia are common disorders impacting quality of life. This population-based, pharmacovigilance study aims to identify systemic drugs disproportionately linked to these disorders.
Methods
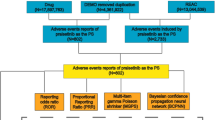
Data from the Food and Drug Administration Adverse Event Reporting System (FAERS) were analysed (Q4 2003 to Q2 2024). Disproportionality analyses were conducted to identify drugs with ≥10 primary suspect reports for which cases of blepharitis, MGD, or chalazion were overreported, using reporting odds ratios (RORs).
Results
1923 blepharitis, 202 MGD, and 290 chalazion reports were identified. MGD was overreported for finasteride (ROR = 71.6, 95% CI = 37.9–135.3), while chalazion was overreported for bortezomib (ROR = 73.9, 95% CI = 51.4–106.2). All three conditions were overreported for dupilumab (blepharitis: ROR = 35.7, 95% CI = 22.8–55.9; MGD: ROR = 15.4, 95% CI = 7.3–32.5; chalazion: ROR = 12.6, 95% CI = 5.6–28.5). Safety signals, predominantly associated with blepharitis, were also identified for isotretinoin, docetaxel, panitumumab, cetuximab, tretinoin, alendronate, erlotinib, zoledronate, daxibotulinumtoxinA, and infliximab.
Conclusions
This pharmacovigilance study identified associations between several systemic medications with reports of blepharitis, MGD, and chalazion. MGD and chalazion were overreported for finasteride and bortezomib, respectively, whereas all three conditions were overreported for dupilumab. These findings highlight systemic medications as often overlooked contributors to localised eyelid inflammation. Nonetheless, these drugs are known to reduce morbidity and mortality across many diseases. Therefore, while risks may not necessarily outweigh benefits to warrant changes in prescribing practices, clinicians should remain vigilant for such side effects, particularly in patients at higher risk, and to consider prophylactic measures when appropriate, such as educating patients about eyelid hygiene and counselling them to promptly report symptoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Further enquiries can be directed to the corresponding author.
References
Kudasiewicz-Kardaszewska A, Grant-Kels JM, Grzybowski A. Meibomian gland dysfunction and blepharitis: a common and still unsolved ophthalmic problem. Clin Dermatol. 2023;41:491–502.
Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40:343–67.
Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–64.
Evans J, Vo KBH, Schmitt M. Chalazion: racial risk factors for formation, recurrence, and surgical intervention. Can J Ophthalmol. 2022;57:242–6.
Fadini GP, Avogaro A. SGTL2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 2017;5:680–1.
Dores GM, Bryant-Genevier M, Perez-Vilar S. Adverse events associated with the use of sipuleucel-T reported to the US Food and Drug Administration’s adverse event reporting system, 2010-2017. JAMA Netw Open. 2019;2:e199249.
Zhou C, Peng S, Lin A, Jiang A, Peng Y, Gu T, et al. Psychiatric disorders associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database. EClinicalMedicine. 2023;59:101967.
Cortes J, Mauro M, Steegmann JL, Saglio G, Malhotra R, Ukropec JA, et al. Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: data from the FDA Adverse Event Reporting System. Am J Hematol. 2015;90:E66–E72.
McGwin G, Maclennan P, Owsley C. Association between pentosan polysulfate sodium and retinal disorders. JAMA Ophthalmol. 2022;140:37–42.
Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharm. 2011;72:905–8.
Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6.
Bate A. Bayesian confidence propagation neural network. Drug Saf. 2007;30:623–5.
Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67:75–93.
Nguyen LH, Makino A, Namkoong P, Yiannakou Y, Narain K. Finasteride-induced clinical ocular toxicity. Invest Ophthalmol Vis Sci. 2018;59:2655.
Traish AM. Health risks associated with long-term finasteride and dutasteride use: it's time to sound the alarm. World J Men’s Health. 2020;38:323–37.
Zakrzewska A, Wiącek MP, Słuczanowska-Głąbowska S, Safranow K, Machalińska A. The effect of oral isotretinoin therapy on meibomian gland characteristics in patients with acne vulgaris. Ophthalmol Ther. 2023;12:2187–97.
Fraunfelder FT, Fraunfelder FW, Edwards R. Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol. 2001;132:299–305.
Zhang P, Tian L, Bao J, Li S, Li A, Wen Y, et al. Isotretinoin impairs the secretory function of meibomian gland via the PPARγ signaling pathway. Invest Ophthalmol Vis Sci. 2022;63:29.
Tanriverdi C, Nurozler Tabakci B, Donmez S. Longitudinal assessment of meibomian glands and tear film layer in systemic isotretinoin treatment. Eur J Ophthalmol. 2022;32:885–93.
Gupta S, Silliman CG, Trump DL. Docetaxel-induced meibomian duct inflammation and blockage leading to chalazion formation. Prostate Cancer Prostatic Dis. 2007;10:396–7.
Fraunfelder FW, Yang HK. Association between bortezomib therapy and eyelid chalazia. JAMA Ophthalmol. 2016;134:88–90.
Veys MC, Delforge M, Mombaerts I. Treatment with doxycycline for severe bortezomib-associated blepharitis. Clin Lymphoma Myeloma Leuk. 2016;16:e109–e112.
Paravathaneni M, Thota V, Mulla S, Thirumaran R, Thar YY. A case report on bortezomib-induced bilateral chalazion. Cureus. 2020;12:e10062.
Gallenga CE, Mura M, Gallenga PE. Suggestions on gut-eye cross-talk: about the chalazion. Int J Ophthalmol. 2022;15:1566.
Vingopoulos F, Lazzaro DR. Dupilumab-associated blepharoconjunctivits with giant papillae. Int Med Case Rep. J. 2020;13:303–5.
Levine RM, Tattersall IW, Gaudio PA, King BA. Cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018;154:1485–6.
Popiela MZ, Barbara R, Turnbull AMJ, Corden E, Martinez-Falero BS, O’Driscoll D, et al. Dupilumab-associated ocular surface disease: presentation, management and long-term sequelae. Eye. 2021;35:3277–84.
Wang Y, Jorizzo JL. Retrospective analysis of adverse events with dupilumab reported to the United States Food and Drug Administration. J Am Acad Dermatol. 2021;84:1010–4.
Foley P, Kerdraon YA, Hogden JP, Shumack S, Spelman L, Sebaratnam DF, et al. Dupilumab-associated ocular surface disease: an interdisciplinary decision framework for prescribers in the Australian setting. Australasian J Dermatol. 2022;63:421–36.
Medicines and Healthcare products Regulatory Agency. Dupilumab (Dupixent▼): risk of ocular adverse reactions and need for prompt management. 2022. Available at: https://www.gov.uk/drug-safety-update/dupilumab-dupixentv-risk-of-ocular-adverse-reactions-and-need-for-prompt-management#dupilumab.
Author information
Authors and Affiliations
Contributions
Dana Taghaddos was responsible for interpreting results and writing the manuscript. Andrew Mihalache was responsible for the conception of the study’s design, analysing data, interpreting results, and writing the manuscript. Ryan S. Huang was responsible for analysing data and interpreting results. Marko M Popovic was responsible for revision of the manuscript and supervision of the study. Clara C. Chan was responsible for revision of the manuscript and supervision of the study. All authors fulfil ICMJE Criteria for authorship.
Corresponding author
Ethics declarations
Competing interests
DT: None; AM: None; RSH: None; MMP: Financial support (to institution)—PSI Foundation, Fighting Blindness Canada; CCC: Consultant—Aequus, Abbvie, Admare Bioinnovations, Allergan Inc., Aurion, Bausch & Lomb, Johnson & Johnson Vision, Kala Ophthalmics, Labtician Ophthalmics Inc., Novartis, Santen Inc., Sun Ophthalmics, Thea, Valeo Pharma. Shareholder—Abbott, Pfizer. Financial support (to institution)—Aurion, Corneat, Claris Bio. Lecture fees—Abbvie, Allergan Inc., Johnson & Johnson Vision, Labtician Ophthalmics Inc., Thea.
Ethical approval
An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taghaddos, D., Mihalache, A., Huang, R.S. et al. Drugs linked to blepharitis, meibomian gland dysfunction, and chalazion: a real-world, population-based pharmacovigilance analysis. Eye 39, 2912–2917 (2025). https://doi.org/10.1038/s41433-025-03985-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03985-x
This article is cited by
-
Drug-associated inflammatory eye lid disorders often underrecognised
Reactions Weekly (2025)