Abstract
Purpose
To identify clinical phenotypes in pachychoroid disease (PD), characterise long-term progression patterns, and evaluate the effect of photodynamic therapy (PDT) using machine learning and longitudinal probabilistic modelling.
Methods
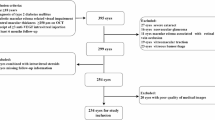
This retrospective cohort study included 973 eyes from 663 patients (mean age 54.4 ± 12.8 years; 80% male) diagnosed with PD. Eyes were classified based on macular fluid status into no history, primary, recurrent, or resolved fluid categories. Multimodal imaging data were analysed at baseline and at the final follow-up visit ( > 5 years). Phenotypic clusters were identified using cross-validated K-means clustering on imaging and clinical features. Longitudinal changes in phenotype were evaluated with Markov modelling, including the effect of photodynamic therapy (PDT) on cluster transitions. Visual acuity (VA) changes were estimated with linear mixed models.
Results
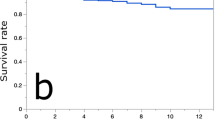
Four distinct clusters were defined: Cluster 1 (233 eyes) consisted of younger patients exhibiting active fluid and mild structural alterations (LogMAR 0.16); Cluster 2 (317 eyes) represented mild or resolving PD with optimal VA (LogMAR 0.05); Cluster 3 (336 eyes) featured chronic fluid accumulation, significant structural damage, and moderate visual impairment (LogMAR 0.35); Cluster 4 (87 eyes) corresponded to severe bilateral disease and worst VA (LogMAR 0.66). At baseline, Clusters 1 to 4 were distributed as follows: 22.7%, 25.5%, 39.8%, and 12.0%. Over follow-up (mean 90.2 ± 24 months), the distribution shifted to 3.7%, 32.4%, 36.6%, and 27.3%, indicating progression toward more advanced phenotypes. Cluster 1 had frequent transitions, with 53% eyes progressing to Cluster 2 and 37% to Cluster 3. Cluster 4 showed minimal transition (96% stable). Interaction indicated greater visual deterioration in more severe baseline phenotypes (p = 0.01). PDT administration did not significantly alter disease progression (p > 0.5).
Conclusion
PD exhibits distinct, dynamically evolving phenotypes with measurable probabilistic progression over time. As this represents an exploratory, data-driven analysis, the identified clusters should be interpreted as hypothesis-generating. Significant structural changes occurred despite PDT, underscoring the need for therapies capable of modifying the underlying disease course rather than its local manifestations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Cheung CMG, Dansingani KK, Koizumi H, Lai TYY, Sivaprasad S, Boon CJF, et al. Pachychoroid disease: review and update. Eye. 2025;39:819–34.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–88.
Ersoz MG, Demirel S, Iovino C, Demirel S, Chhablani JK. Is pachychoroid serous retinopathy a better name to describe the features of central serous chorioretinopathy? Retina. 2024;44:1475–7.
Ng DS, Ho M, Chen LJ, Yip FL, Teh WM, Zhou L, et al. Optical coherence tomography angiography compared with multimodal imaging for diagnosing neovascular central serous chorioretinopathy. Am J Ophthalmol. 2021;232:70–82.
Borrelli E, Battista M, Sacconi R, Gelormini F, Querques L, Grosso D, et al. OCT risk factors for 3-year development of macular complications in eyes with “resolved” chronic central serous chorioretinopathy. Am J Ophthalmol. 2021;223:129–39.
Mohabati D, Boon CJF, Yzer S. Risk of recurrence and transition to chronic disease in acute central serous chorioretinopathy. Clin Ophthalmol. 2020;14:1165–75.
Zola M, Chatziralli I, Menon D, Schwartz R, Hykin P, Sivaprasad S. Evolution of fundus autofluorescence patterns over time in patients with chronic central serous chorioretinopathy. Acta Ophthalmol. 2018;96:e835–9.
Pauleikhoff LJB, Diederen RMH, Chang-Wolf JM, Moll AC, Schlingemann RO, van Dijk EHC, et al. Choroidal hyperpermeability patterns correlate with disease severity in central serous chorioretinopathy: CERTAIN study report 2. Acta Ophthalmol. 2024;102:e946–55.
Viggiano P, Boscia G, Sadeghi E, Cheung G, Borrelli E, Alessio G, et al. Pachychoroid disease spectrum: how multimodal imaging and OCT angiography have improved our knowledge. Prog Retin Eye Res. 2025;107:101372.
Siedlecki J, Schworm B, Priglinger SG. The pachychoroid disease spectrum-and the need for a uniform classification system. Ophthalmol Retina. 2019;3:1013–5.
Chhablani J, Behar-Cohen F. Central Serous Chorioretinopathy International G. Validation of central serous chorioretinopathy multimodal imaging-based classification system. Graefes Arch Clin Exp Ophthalmol. 2022;260:1161–9.
Chhablani J, Cohen FB. Central serous chorioretinopathy international G. multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retina. 2020;4:1043–6.
Singh SR, Iovino C, Zur D, Masarwa D, Iglicki M, Gujar R, et al. Central serous chorioretinopathy imaging biomarkers. Br J Ophthalmol. 2022;106:553–8.
Lindenberg S, Mahmoudi A, Oncel D, Corradetti G, Oncel D, Emamverdi M, et al. Acquired vitelliform lesions in intermediate age-related macular degeneration: a cross sectional study. Ophthalmol Retina. 2024;8:854–62.
Mohabati D, Hoyng CB, Yzer S, Boon CJF. Clinical characteristics and outcome of posterior cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2020;40:1742–50.
Sato-Akushichi M, Kinouchi R, Ishiko S, Hanada K, Hayashi H, Mikami D, et al. Population-based prevalence and 5-year change of soft drusen, pseudodrusen, and pachydrusen in a Japanese population. Ophthalmol Sci. 2021;1:100081.
Spaide RF, Ryan EH Jr. Loculation of fluid in the posterior choroid in eyes with central serous chorioretinopathy. Am J Ophthalmol. 2015;160:1211–6.
Hansraj S, Chhablani J, Behera UC, Narula R, Narayanan R, Sahoo NK, et al. Inner choroidal fibrosis: an optical coherence tomography biomarker of severity in chronic central serous chorioretinopathy. Am J Ophthalmol. 2024;264:17–24.
Mirshahi R, Naseripour M, Shojaei A, Hosoda Y, Takahashi A, Muraoka Y, et al. Differentiating a pachychoroid and healthy choroid using an unsupervised machine learning approach. Sci Rep. 2022;12:16323.
Yagi M, Miyake M, Mori Y, Hosoda Y, Takahashi A, Muraoka Y, et al. Natural course of pachychoroid pigment epitheliopathy. Ophthalmol Sci. 2022;2:100201.
Sahoo NK, Ong J, Selvam A, Avdalimov M, Gujar R, Lupidi M, et al. Ten-year follow-up and sequential evaluation of multifocal retinal pigment epithelium abnormalities in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2023;261:1883–91.
Bartolucci F, Pandolfi S, Pennoni F. LMest: an R package for latent Markov models for longitudinal categorical data. J Stat Softw. 2017;81:1–38.
Ravenstijn M, van Dijk EHC, Haarman AEG, Kaden TR, Vermeer KA, Boon CJF, et al. Myopic presentation of central serous chorioretinopathy. Retina. 2021;41:2472–8.
Spaide RF. The ambiguity of pachychoroid. Retina. 2021;41:231–7.
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118.
Cicinelli MV, Barlocci E, Giuffre C, Rissotto F, Introini U, Bandello F. Integrating machine learning and traditional survival analysis to identify key predictors of foveal involvement in geographic atrophy. Investig Ophthalmol Vis Sci. 2024;65:10.
Yoneyama S, Fukui A, Sakurada Y, Terao N, Shijo T, Kusada N, et al. Distinct characteristics of simple versus complex central serous chorioretinopathy. Retina. 2023;43:389–95.
Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–72.
Sato Y, Ueda-Arakawa N, Takahashi A, Miyake M M, Mori Y, Miyara Y, et al. Clinical characteristics and progression of pachychoroid and conventional geographic atrophy. Ophthalmol Sci. 2024;4:100528.
Toto L, Ruggeri ML, Evangelista F, Viggiano P, D' Aloisio R, De Nicola C, et al. Choroidal modifications assessed by means of choroidal vascularity index after oral eplerenone treatment in chronic central serous chorioretinopathy. Eye. 2023;37:1214–8.
Acknowledgements
a. Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. b. Financial disclosures: Maria Vittoria Cicinelli, Lorenzo Bianco, Lorenzo Caminada, Eugenio Barlocci, Chiara Giuffré, Maria Pia De Carlo, Jay Chhablani, Ugo Introini: No financial disclosures. Francesco Bandello, consultant for: Allergan Inc (Irvine, California, USA), Bayer Shering-Pharma (Berlin, Germany), Hoffmann-La-Roche (Basel, Switzerland), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA), Boehringer-Ingelheim, Fidia Sooft, Ntc Pharma, Sifi. c. Other acknowledgments: None.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception or design of the work, the acquisition, analysis, and interpretation of data, drafting of the work, revising it critically for intellectual content. Each of the coauthors has seen and agrees with the way his or her name is listed.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of generative AI technologies in the writing process
During the preparation of this work, the authors used ChatGPT4 to improve the readability and language of the manuscript. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cicinelli, M.V., Bianco, L., Caminada, L. et al. Towards an objective classification of pachychoroid disease and its risk of progression. Eye (2026). https://doi.org/10.1038/s41433-026-04286-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41433-026-04286-7