Abstract
Several studies have reported a positive association between psychological stress and cardiovascular diseases; however, there is scarce evidence about various aspects of life stress, including traumatic, positive, and negative events. We aimed to evaluate the association between various stressful life events and indicators of cardiovascular risk, including the augmentation index. A total of 3276 participants from the Cardiovascular and Metabolic Diseases Etiology Research Center cohort (Mean age: 50.9) were analyzed cross-sectionally. By using the Life Experience Questionnaire, exposures were grouped as a “positive event,” “negative event,” or “traumatic event.” The augmentation index and subclinical atherosclerosis were measured. Multivariate polytomous logistic regression was used. Overall, stressful life events did not show any significant association with any cardiovascular index; however, increased odds ratios were observed between augmentation index quartiles and those who had experienced traumatic events (quartile 4: odds ratio = 1.41, 95% confidence interval = 1.09–1.82). The association remained valid among women when stratified by sex. There was no significant result in men. Traumatic events in women were positively associated with the augmentation index. These findings suggest that more attention should be paid to trauma in the context of increased cardiovascular risk in women.
Similar content being viewed by others
Introduction
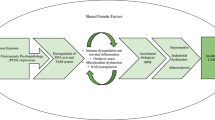
Psychological stress has been heavily reported as a contributing factor to cardiovascular disease (CVD) [1]. Similar to longitudinal studies focusing on childhood distress associated with certain cardiovascular outcomes [2, 3], studies in adults have shown that stressful life events in adulthood increase the risk of CVD onset [4]. The association between stress and CVD can be partially explained by a feeling of frustration and by tension-induced cardiovascular outcomes such as myocardial ischemia [5]. Reactions to stressful life events can manifest in various ways, and they can be experienced as positive versus negative events or traumatic versus nontraumatic events. Concerning mental health outcomes, two previous studies suggested that negative life events could be indicative of other psychiatric symptoms [6, 7], whereas several studies reported that exposure to psychological trauma was associated with increased risk of CVD events [8, 9].
In the literature, chronic mental stress has been associated with coronary artery disease [10], and acute stress has been associated with left ventricular dysfunction [11] and myocardial ischemia [12]. Arterial stiffness is known to be one of the initial processes leading to CVD [13, 14], and large-artery stiffness is an important risk factor for left ventricular dysfunction [15]. One study highlighted the role of mental stress, including childhood trauma and negative life events, on arterial stiffness; among 650 participants from the Netherlands, the authors found an increased likelihood of an association between recent negative life events and increased central arterial stiffness [16]. However, they did not assess the effect of positive life events or recent trauma on cardiovascular indices, including arterial stiffness, and atherosclerosis. Furthermore, most of the studies were conducted in Western populations, and there have been no corresponding studies conducted in the Asian population thus far.
The average population of the Republic of Korea (hereafter, Korea) has been reported to show intensely high levels of mental stress, with Korea having the highest suicide rate among the member nations of the Organization for Economic Cooperation and Development [17]. In this study, we aimed to evaluate the association between different types of life events (including events that are traumatic or involve positive or negative stress) and indicators of subclinical CVD, such as the augmentation index and carotid atherosclerosis, in the Korean population.
Materials and methods
Study population
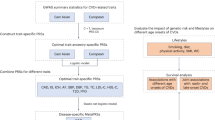
This study utilized the baseline data of the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) cohort. Between 2013 and 2016, a total of 3332 participants aged 30–64 years were recruited for this study. The CMERC cohort initially aimed to enroll 8000 community-living individuals who were free from CVDs such as myocardial infarction and heart failure. Women were targeted for inclusion twice as frequently as men, since cardiovascular outcomes are generally twice as frequent in men than women. Participants reflected the characteristics of the urban middle class in South Korea; compared with people enrolled in the Korea National Health and Nutritional Examination Survey, who were selected as a nationally representative sample in 2013–2014, CMERC cohort participants were more likely to be female and older, be more highly educated, have higher income, be white-collar workers, and follow a healthier lifestyle pattern [18]. Among them, we excluded 54 persons with missing values from cardiovascular measurements, such as the radial augmentation index or carotid ultrasonography parameters. Furthermore, two persons who failed to answer any item in the Life Experience Survey (LES) questionnaire were also excluded. A total of 3276 participants (1135 men and 2141 women) were finally included in the analysis (Fig. 1).
Measurement of stressful life events and traumatic events
In the CMERC, trained interviewers used the LES questionnaire [19], translated into Korean and validated for the Korean population [20], to investigate stressful life events that had occurred in the previous 6 months among the participants. The questionnaire contains 57 life event items, including marital status, death of a close family member, sexual difficulties, or trouble with an employer. (Supplementary Box 1) Of the 57 events, 10 items, mostly regarding the hardships of student life, were omitted in this study, resulting in 47 questions in the final survey [18]. If the participants reported having experience with the corresponding item, they were asked to score the item on a 7-point scale ranging from +3 (extremely positive experience) to −3 (extremely negative experience). Trained interviewers administered the survey. Among the 47 items, we selected eight items (Supplementary Box 2, please see http://hyper.ahajournals.org) that were relevant to the 16-item Brief Trauma Questionnaire [21, 22] and grouped them as “traumatic events.” The 16-item Brief Trauma Questionnaire is known to be a valid and reliable assessment tool for trauma exposure [22]. Participants who responded to any item in the LES questionnaire with a positive impact score were grouped as the “positive event group,” whereas those who responded to any item with a negative impact score were categorized as the “negative event group.” We also summed the impact scores in each separate category.
Covariates
In the baseline assessment, the participants were asked about their demographic characteristics, medical history, family history, behavioral factors, sleep duration, and sleep quality using a general questionnaire with a standardized protocol. We applied the Beck Depression Index (BDI), translated and validated in Korean [23], to assess depressive symptoms. The BDI includes 21 items on a Likert scale from 0 to 3 regarding the occurrence of symptoms related to depression in the previous 2 weeks. A higher score represents more serious impairment. Body size and composition measurements and cardiovascular examinations, such as central blood pressure, carotid artery ultrasonography, and radial pulse tonometry, were conducted by trained personnel with a standardized protocol, along with assessments of biochemical indicators. Detailed information can be found elsewhere [18].
Years of education were grouped as <6, 6–8, 9–11, and ≥12 years, based on the education system in Korea. Family income was grouped into <$36,000, $36,000 to <60,000, $60,000 to <84,000, and ≥$84,000. Marital status was categorized into “never married,” “married and living with spouse,” “married but living separately,” and “widowed.” We categorized the participants who reported a history of stroke, transient ischemic stroke, myocardial infarction, angina pectoris, and heart failure into the “CVD group”. In addition, participants who reported a parental history of stroke or myocardial infarction were included in the group with a family history of CVD. Sleep duration was divided into quartiles.
Measurement of augmentation index and carotid intima-media thickness (IMT)
We noninvasively assessed the radial augmentation index [24]. The augmentation index has been used to measure arterial stiffness with respect to cardiovascular risk, especially in middle-aged and older populations. The radial augmentation index has been reported to be related to hypercholesterolemia [25] and the Framingham risk score [26].
Well-trained technicians used a HEM-9000AI (Omron Healthcare, Kyoto, Japan), a radial pulse waveform analyzer, to measure and calculate the augmentation index. The sensor of this device is placed over the radial artery on the left wrist, and the artery is flattened automatically by the machine. The device modifies the applanation constraint pressure, and an optimal sensing element is selected to record the appropriate pulse wave. Inside the pulse measurement unit, the digitalized pressure signals are adjusted at 500 Hz and transduced to a computing unit. By using the amplitude ratio of the late systolic peak to the early systolic peak, the augmentation index was computed as in the same way in the previous methods [27]. The value was adjusted to a heart rate of 75 beats per minute and divided into quartiles for each sex.
We applied carotid artery ultrasonography using an identical device (Accuvix SG; Samsung Medison, Seoul, Korea) for all participants in the study. From the proximal common carotid arteries to the bulb region, the carotid IMT was scanned with a 1-cm interval at the time of the R-wave in the electrocardiogram. We measured both the right and left sides of the carotid artery and measured the maximum/mean value of the carotid IMT. If the thickness of the carotid IMT was ≥1.5 mm or if it increased by ≥50% compared with the surrounding IMT, the presence of plaque was considered. We measured the right and left common artery, internal carotid artery, external carotid artery, and bulb area. The IMT was divided into quartiles based on the population distribution from each sex. The interrater reliability of carotid IMT measurements was estimated to be high, and the agreement of carotid plaque measurement was fair [28].
Statistical analyses
The baseline differences in the characteristics of participants with low and high frequencies of overall stressful life events were analyzed using the chi-square test for categorical variables and the t-test for continuous variables. Comparisons between trauma-exposed and nonexposed groups in the prior 6 months were also conducted in the same way.
The association between stressful life events, including traumatic, positive, and negative events, and the augmentation index was examined using multivariate polytomous logistic regression and was adjusted for age, sex, marital status, education, income, height, systolic blood pressure, body mass index, cigarette smoking, alcohol drinking, physical activity, history of CVD, and family history of CVD. In addition, we tested whether the association between life events and the augmentation index differed by age group by limiting the analysis to those younger than age 50 and by adding an interaction term between life events and age groups, which was divided by the median age of the overall population (53 years). To assess the mediating property of depressive symptoms between stressful life events and the augmentation index, we further adjusted for the BDI. To evaluate the association between stressful life events and carotid IMT, we used polytomous logistic regression, adjusting for age, sex, marital status, education, income, body mass index, cigarette smoking, alcohol drinking, physical activity, history of CVD, and family history of CVD.
Since the augmentation index can be affected by systolic blood pressure [29] and measuring the augmentation index can be affected by limb length [30], we adjusted for the mean systolic blood pressure and height that were separately added in the final model.
Results
The overall population had a mean age of 50.9 (SD 9.6), and 65.4% were women. Approximately 50.3% of men and 47.8% of women had experienced three or more stressful life events during the 6 months prior to the survey. In the baseline assessment, younger participants reported higher numbers of stressful life events and trauma experiences in the previous 6 months (Supplementary Table 1). Participants with higher education, any comorbidity, a family history of CVD or cerebrovascular disease, or alcohol use tended to be exposed to more stressful life events and traumatic events. Inversely, participants with a personal history of CVD were less likely to have experienced stressful life events. Those with stressful life events and traumatic events showed increased BDI scores (Table 1).
Table 2 and Supplementary Table 2 show the associations between different types of stressful life events, including traumatic, positive, and negative events, and the augmentation index. No subcategory of stressful life events was associated with systolic or diastolic blood pressure, and the results were similar when restricting the population to age 50 or under (Supplementary Table 4). Similarly, there was no significant association between the augmentation index and overall stressful life events, positive events, or negative events; however, we observed significantly increased odds ratios (ORs) between traumatic events and the augmentation index, and the ORs increased linearly as the range of the augmentation index increased; this trend remained in the sensitivity analysis, limiting the sample to participants age 50 and under (Supplementary Table 4). When we stratified the results by sex (Table 3), only women showed increased ORs in the trauma-exposed group. In addition, significantly increased ORs were observed between the negative event-exposed group in women and the augmentation index range in the third and fourth quartiles. When the interaction term between age and each life event was added to the model, there were significant results for negative events and age in men and for traumatic events and age in women, suggesting that an increased augmentation index after these events would likely occur later in life (results not shown). However, the overall trend was similar to those in the sensitivity analysis after restricting the population to age 50 or under (Supplementary Table 5). BDI scores were additionally adjusted in the final model, and women with trauma showed slightly attenuated ORs in each augmentation index strata, but the result was still statistically significant. In men, the overall results were not significant.
Participants exposed to positive life events showed a lower likelihood to exhibit certain strata in the mean IMT (second and fourth quartiles); however, there were no significant results between overall stressful life events and carotid ultrasonography measurements (Supplementary Table 3, please see http://hyper.ahajournals.org).
Discussion
In this study, we observed a positive association between traumatic events and an elevated augmentation index in women. However, men were less likely to show an association between negative events and the augmentation index as augmentation increased. We did not observe significant associations between the augmentation index and overall stressful life events or positive events. IMT was not associated with any type of stressful life event. Depressive symptoms may partially explain the association between trauma and an increased augmentation index.
In a previous study of 650 Dutch participants, childhood and recent mental stress were assessed in association with both carotid arterial atherosclerosis and central arterial stiffness using the augmentation index [16]. In other studies, an increased augmentation index was observed in a population that had experienced daily life stress (β = 0.06, p = 0.02 per standard deviation increase), measured with the 20-item Daily Hassles Questionnaire [31], and negative life events (β = 0.07, p = 0.01 per standard deviation increase), measured with the list of Threatening Events Questionnaire [32]. The authors found no significant associations between indicators of stressful life events and carotid atherosclerosis. As in our study, the association between life stress and the augmentation index was partially mediated by depressive symptoms, which is consistent with the results of other previous research [2, 3, 33]. However, in the abovementioned study in Dutch participants, the authors did not separately consider the effect of trauma in adulthood. Instead, a number of the studies evaluating the role of mental stress on cardiovascular risk factors used a mental arithmetic test, which was a more experimental exposure, to induce perceived stress [34]. In a study using a randomized crossover design, pulse wave velocity was associated with an increased augmentation index and with mental stress among 19 healthy participants [35].
In addition, only women were more likely to show an association between trauma and the augmentation index in our study. Rich-Edwards et al. suggested that women have an increased risk of adverse cardiovascular events due to the higher levels of exposure to physical and sexual abuse [3]. Furthermore, there was no significant association between stressful life event domains and carotid atherosclerosis. This result is consistent with several other studies [16, 36], and DeCara suggested that not all arterial beds are affected by subclinical CVD in a constant manner [37]. In men, we did not observe an overall significant result before or after adjusting for depression as an additional covariate among any category of stressful life events. We were able to observe a possible tendency toward decreasing OR magnitude among negative event-exposed men and the highest quartile augmentation index after adjusting for depression. A study [38] conducted in middle-aged men suggested a significant association between depression and the augmentation index, albeit there was no significant result among women. In our findings, we carefully suggest that the association between negative life events and the augmentation index could be considerably mediated by depression symptoms, and the acute stress from negative events might temporally decrease the augmentation index in men; Laurent et al. [39] suggested that cyclic stress may cause arterial remodeling through a decrease in arterial stiffness.
Stress is a well-known risk factor for CVD, and it can manifest in various forms. Frequently, life events precede mental stress, and they can be a traumatic or nontraumatic experience or a positive or negative event. Several studies proposed that negative life events can contribute to adverse cardiovascular events; [40] however, recent studies refuted this idea, saying that negative life events would not meet the Diagnostic and Statistical Manual (DSM)-IV/DSM-5 criteria as an indicator of various health outcomes [41, 42]. In our study, those who had been exposed to traumatic events showed an increased likelihood to have an increased augmentation index, and this result has not been reported previously. Numerous population-based studies have evaluated the association between trauma and increased CVD among persons who were exposed to trauma, including patients with posttraumatic stress disorder [9, 43]. In those studies, a number of mediating mechanisms were suggested, such as increased inflammatory biomarkers; stress on autonomic function; changes in the renin–angiotensin system and hypothalamic–pituitary–adrenal axis; and stimulation of the sympathetic-adrenal-medullary system, causing increased levels of catecholamine and other hormones [44, 45].
However, our results showed that experiencing a negative life event in the previous 6 months seems to reduce the likelihood of high systolic blood pressure, and this finding was consistent with several previous findings [46,47,48] In these studies, stressful life events showed a negative association with systolic blood pressure, and it is suggested that stressful life events could blunt cardiac reactivity and not show elevated blood pressure [46]. This phenomenon was found to be related to certain factors, such as gender or social network size. Some researchers proposed that this blunting effect could occur with exposure to subjective low-impact stress or with the use of effective coping strategies developed from previous negative life events [48, 49]. However, this result could also be explained by the baseline characteristics of the participants that had experienced stressful events; they tended to be younger, have higher education levels, have fewer comorbidities and less prevalent CVD, and spend more time performing physical activity. Although we controlled for these confounders, their residual effects might be still present in the results. In further analyses, stratifying by social network size and specifying information of negative life events with regard to severity or timing should be considered.
Our results can be explained by several mechanisms. Exposure to trauma and negative life events can lead to unhealthy behaviors, such as cigarette smoking or excessive alcohol consumption [50], and these factors can affect arterial stiffness [51] Nevertheless, after adjusting for lifestyle factors such as cigarette smoking, alcohol consumption, and physical activity, the association between trauma and the increased augmentation index remained significant. In our study, we observed that the association was partially attenuated after adjusting for depressive symptoms, which may indicate a potential mediating role of depression between trauma and the development of an increased augmentation index. In response to traumatic stress, cortisol is released, activating the sympathetic nervous system, which can affect arterial stiffness [52].
This study has several limitations. First, because we utilized the baseline data of the CMERC cohort, we performed a cross-sectional analysis; we were restricted in inferring a definite causal association between stressful life events and increased arterial stiffness. Second, we utilized the radial augmentation index, which is a relatively indirect method of measuring large-artery stiffness, as this procedure uses applanation tonometry; however, the prognostic value of this method has been evaluated previously [13]. Third, although we also adjusted for various factors, including demographic and lifestyle factors, family history, and comorbidity, our analyses may still exhibit residual confounding. Fourth, it is possible that some of the results were due to chance; however, we found a strong dose-response pattern in women with trauma exposure after adjusting for various covariates. Fifth, the augmentation index does not fully represent arterial stiffness because it is determined not only by aortic stiffness but also by heart rate, blood pressure, height and peripheral vascular resistance. Finally, we made a new operational category for trauma with 8 items in the LES; however, this category may not capture the entire spectrum of clinical trauma, and further evaluation is warranted.
Despite these limitations, this study has considerable strengths. To our knowledge, this is the first Asian study to evaluate life stress considering trauma and its association with arterial stiffness. Compared with a previous study [16], the current study included a larger number of participants, with detailed information on various items related to cardiovascular health. We were able to assess the role of stressful life events in four different aspects and considered the role of depressive symptoms using a validated instrument.
Perspectives
We suggest that exposure to traumatic events may increase arterial stiffness in women, and this can consequently induce the onset of clinical CVD. Varied aspects of stressful life events showed different patterns of arterial stiffness increases; compared with other stressful life events, traumatic experience showed a high correlation with increased arterial stiffness, and this result may provide a clue for understanding the pathophysiology of CVD in women with trauma and posttraumatic stress disorder. More attention should be paid to trauma exposure in women in terms of arterial stiffness and CVD. Although we were not able to establish direct causation between stressful life events and arterial stiffness due to temporality, further studies with a longitudinal design with a wider assessment of exposure to traumatic events and arterial stiffness are warranted.
Novelty and significance
What is new
-
This is the first study to examine life stress considering trauma and its association with arterial stiffness in an Asian population.
What is relevant
-
Arterial stiffness has been known to be one of the initial processes leading to CVD and is also related to hypertension.
-
We examined the association between traumatic events, negative life events and arterial stiffness, which can also be an initiating factor for hypertension.
Summary
-
Traumatic events in women were positively associated with the augmentation index, and we suggest that more attention should be paid to trauma in the context of increased cardiovascular risk in women.
References
Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–46.
Korkeila J, Vahtera J, Korkeila K, Kivimäki M, Sumanen M, Koskenvuo K, et al. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303.
Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126:920–7.
Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500.
Gullette EC, Blumenthal JA, Babyak M, Jiang W, Waugh RA, Frid DJ, et al. Effects of mental stress on myocardial ischemia during daily life. JAMA. 1997;277:1521–6.
Cameron A, Palm K, Follette V. Reaction to stressful life events: what predicts symptom severity? J Anxiety Disord. 2010;24:645–9.
Furniss T, Beyer T, Müller JM. Impact of life events on child mental health before school entry at age six. Eur child Adolesc psychiatry. 2009;18:717–24.
Hendrickson CM, Neylan TC, Na B, Regan M, Zhang Q, Cohen BE. Lifetime trauma exposure and prospective cardiovascular events and all-cause mortality: findings from the Heart and Soul Study. Psychosom Med. 2013;75:849–55.
Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–9.
Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217.
Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. New Engl J Med. 1988;318:1005–12.
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. New Engl J Med. 2005;352:539–48.
Laurent S. European network for non-invasive investigation of large arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Yannoutsos A, Ahouah M, Tubiana CD, Topouchian J, Safar ME, Blacher J. Aortic stiffness improves the prediction of both diagnosis and severity of coronary artery disease. Hypertens Res. 2018;41:118–25.
Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–93.
Bomhof-Roordink H, Seldenrijk A, van Hout HP, van Marwijk HW, Diamant M, Penninx BW. Associations between life stress and subclinical cardiovascular disease are partly mediated by depressive and anxiety symptoms. J Psychosom Res. 2015;78:332–9.
OECD. Health at a glance 2013: OECD Indicators, OECD Publishing, Paris. https://doi.org/10.1787/health_glance-2013-en. 2013.
Shim JS, Song BM, Lee JH, Lee SW, Park JH, Choi DP, et al. Cardiovascular and metabolic diseases etiology research center (CMERC) cohort: study protocol and results of the first 3 years of enrollment. Epidemiol Health. 2017;39:e2017016.
Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J consulting Clin Psychol. 1978;46:932.
Lee Y-H. Relations between attributional style, life events, event attribution, hopelessness and depression. Unpublished doctoral dissertation. Seoul: Seoul National University. 1993.
Morgan CA III, Hazlett G, Wang S, Richardson EG Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry. 2001;158:1239–47.
Schnurr P, Vielhauer M, Weathers F, Findler M. The brief trauma questionnaire. White River Junction, VT: National Center for PTSD. 1999.
Kim M, Lee I, Lee C. In female university students sample= the validation study 1 of Korean BDI-2. Korean J Clin Psychol. 2007;26:997–1014.
Weber T, Auer J, O’rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9.
Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–11.
Duprez DA, Kaiser DR, Whitwam W, Finkelstein S, Belalcazar A, Patterson R, et al. Determinants of radial artery pulse wave analysis in asymptomatic individuals. Am J hypertension. 2004;17:647–53.
Kelly R, Hayward C, Avolio A, O’rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–9.
Lee JH, Choi DP, Shim J-S, Kim DJ, Park S-H, Kim HC. Inter-rater reliability of carotid intima-media thickness measurements in a multicenter cohort study. J Health Info Stat. 2016;41:49–56.
Gkaliagkousi E, Douma S. The pathogenesis of arterial stiffness and its prognostic value in essential hypertension and cardiovascular diseases. Hippokratia. 2009;13:70.
Ahn KT, Park K-I, Kim MJ, Oh JK, Han JH, Kwon HJ, et al. Height and sex is strongly associated with radial augmentation index in Korean patients with never-treated hypertension. Clin Interventions Aging. 2016;11:415.
Kanner AD, Coyne JC, Schaefer C, Lazarus RS. Comparison of two modes of stress measurement: daily hassles and uplifts versus major life events. J Behav Med. 1981;4:1–39.
Brugha TS, Cragg D. The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand. 1990;82:77–81.
Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6.
Jern S, Pilhall M, Jern C, Carlsson SG. Short-term reproducibility of a mental arithmetic stress test. Clin Sci. 1991;81:593–601.
Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006;68:231–7.
Seldenrijk A, Van Hout HP, van Marwijk HW, de Groot E, Gort J, Rustemeijer C, et al. Carotid atherosclerosis in depression and anxiety: associations for age of depression onset. World J Biol Psychiatry. 2011;12:549–58.
DeCara JM. Carotid intima-media thickness and risk for coronary heart disease. Curr Cardiovascular Imaging Rep. 2009;2:350.
Onete V, Henry RM, Sep SJS, Koster A, van der Kallen CJ, Dagnelie PC, et al. Arterial stiffness is associated with depression in middle-aged men - the Maastricht Study. J Psychiatr Neurosci. 2018;43:111–9.
Laurent S, Kingwell B, Bank A, Weber M, Struijker-Boudier H. Clinical applications of arterial stiffness: Therapeutics and pharmacology. Am J Hypertension. 2002;15:453–8.
Low CA, Salomon K, Matthews KA. Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom Med. 2009;71:927.
Gold SD, Marx BP, Soler-Baillo JM, Sloan DM. Is life stress more traumatic than traumatic stress? J Anxiety Disord. 2005;19:687–98.
Van Hooff M, McFarlane AC, Baur J, Abraham M, Barnes DJ. The stressor Criterion-A1 and PTSD: a matter of opinion? J Anxiety Disord. 2009;23:77–86.
Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:548–56.
Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–77.
Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309:R315–R321.
Phillips AC, Carroll D, Ring C, Sweeting H, West P. Life events and acute cardiovascular reactions to mental stress: a cohort study. Psychosom Med. 2005;67:384–92.
Boyce WT, Chesterman E. Life events, social support, and cardiovascular reactivity in adolescence. J Dev Behav Pediatr. 1990;11:105–11.
Musante L, Treiber FA, Kapuku G, Moore D, Davis H, Strong WB. The effects of life events on cardiovascular reactivity to behavioral stressors as a function of socioeconomic status, ethnicity, and sex. Psychosom Med. 2000;62:760–7.
Jorgensen RS, Houston BK. Reporting of life events, family history of hypertension, and cardiovascular activity at rest and during psychological stress. Biol Psychol. 1989;28:135–48.
Seelig AD, Rivera AC, Powell TM, Williams EC, Peterson AV, Littman AJ, et al. Patterns of smoking and unhealthy alcohol use following sexual trauma among US Service members. J Trauma Stress. 2017;30:502–11.
Vlachopoulos C, Alexopoulos N, Stefanadis C. Lifestyle modification and arterial stiffness and wave reflections: a more natural way to prolong arterial health. Artery Res. 2006;1:S15–S22.
Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metabol. 2003;88:2527–33.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (grant number 2018R1C1B5083722) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI13C0715).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The protocol of this study was approved by the Institutional Review Board of Yonsei University (YUIRB- 4-2013-0661), and written informed consent was provided by all participants. All procedures in this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jung, S.J., Jeon, Y., Lee, G. et al. Stressful life events and augmentation index: results from the Cardiovascular and Metabolic Diseases Etiology Research Center. Hypertens Res 43, 45–54 (2020). https://doi.org/10.1038/s41440-019-0331-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-019-0331-6