Abstract
Endothelial cells, which regulate arterial stiffness via endothelial-derived substances, are independently and strongly associated with hypertension. However, the exact roles of exosome miRNAs from brain endothelial cells in the development of hypertension are still not fully explored. Here, we investigated the miRNA functions systematically by examining both exosomal small RNA and mRNA of endothelial cells in Wistar Kyoto (WKY) rats versus spontaneously hypertensive rats (SHRs). Our findings revealed that miRNAs, representing ~60–70%, account for the majority of small RNAs. Moreover, we found 159 novel miRNAs in total from the unannotated reads across the diverse samples. Afterwards, 76 differentially expressed miRNAs (37 upregulated, 39 downregulated) and 1709 differentially expressed mRNAs (775 upregulated, 934 downregulated) were identified between SHRs and WKY rats, respectively. Finally, 647 genes targeted by 36 miRNAs came to our attention via identification of the target genes of those abnormal miRNAs. The differentially expressed target genes induced by miRNA changes were mapped to a number of genes involved in various gene functions and pathways. These changes lead to dysregulation of angiogenesis, axonogenesis, neuron-to-neuron synapses, focal adhesion, axon guidance, cell adhesion molecules (CAMs), adherens junction, and ECM-receptor interaction pathways. Together, our study revealed that the miRNAs are changed and contribute to the dysregulated functions and pathways of their target genes and provided more insights into their regulation mechanisms during mammalian hypertension development.
Similar content being viewed by others
Introduction
Hypertension is a complex disease in which blood pressure is sustainably elevated in arteries. It is a major risk factor for heart disease, myocardial infarction, vascular disease, stroke, and chronic kidney disease, since persistent high blood pressure exerts disadvantageous effects on target organs, such as the kidney and heart, resulting in renal disorders and serious cardiovascular diseases [1, 2].
Endothelial cells play a vital role in modulating some arterial properties, including vascular permeability and vascular tone. Endothelial cells regulate arterial stiffness via endothelial-derived substances [3]. Endothelial dysfunction is independently and strongly associated with hypertension [4]. The endothelial damage caused by elevated blood pressure reduces endothelium vasodilation and increases vasoconstriction [5]. The relationships between inflammation, coronary microvascular dysfunction, diminished vasodilator effects, arterial stiffness, and capillary recruitment have also been shown to significantly exist [6, 7]. Inflammation may stiffen the large arteries via its negative impact on endothelial function as a result of reduced nitric oxide (NO) bioavailability and increased activity of opposing mediators such as endothelin-1 [8, 9]. Chronic exposure to a proinflammatory state may likely contribute to remodeling of the microvessels (small arteries and arterioles), structure stiffening on the conduit arteries, impaired endothelial function of the macro- and microvasculature, and further cause elevated blood pressure and target organ damage. Pericytes, the contractile cells within the basement membrane, elongate and wind around the endothelial cells of capillaries throughout the body. They are inserted as an integral part in the basement membrane of the endothelial cells and intercommunicate with them via direct physical contact or paracrine signals, to invigorate and stabilize the maturation process of endothelial cells [5, 10, 11].
MicroRNAs, abbreviated miRNAs, are small endogenous noncoding molecules 18–25 nucleotides long that function in RNA silencing and regulate gene expression at the posttranscriptional level [12]. Many miRNAs are evolutionarily conserved and abundant, which implies that they have important biological functions. Increasing studies have shown that miRNAs play important roles in multiple cellular and molecular events, and their dysregulation in the pathogenesis of various diseases has been found, including hypertension [13, 14].
Exosomes are extracellular vesicles (EVs) that are produced in the endosomal compartment of most eukaryotic cells. They contain a specific cargo of lipids, proteins, and miRNAs [15], which can regulate recipient cell behaviors and could be used as biomarkers for various human diseases. Exosomes are present in tissues and can also be found in biological fluids, including blood and urine. A recent study has proved that miRNAs can be transported into body fluids within exosomes [16]. Once the miRNA is released into the extracellular fluid, exosomes fuse with other cells and transfer the miRNAs to the acceptor cell [17]. For example, Balkom et al. [18] discovered that endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Bovy and colleagues [19] revealed that endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via miRNA transfer.
However, although exosomal miRNAs may have critical roles in cell communication and could be used as biomarkers to detect and monitor disease [20], exosome miRNAs derived from endothelial cells have not been well elucidated for hypertension diagnosis or monitoring. In addition, how exosome miRNAs affect gene functions and which underlying pathways and mechanisms regulate these changes are still not fully characterized. Thus, it remains to be uncovered how exosome miRNAs of endothelial cells mediate gene functions and to what extent these functions exert physiological events in hypertension.
Materials and methods
Sample preparation and identification
Experiments on laboratory animals were authorized by the Laboratory Animal Care and Ethics Committee of the Institute of Microcirculation, Peking Union Medical College & Chinese Academy of Medical Sciences. Wistar Kyoto (WKY) rats (n = 10) and spontaneously hypertensive rats (SHRs) (n = 10) were purchased from Vital River Laboratory Animal Technology Co. Ltd (license no. SCXK 2016–0006, Beijing, China). The brains of rats were immersed in ice-cold isolation buffer immediately after decapitating the rats. After removing the tissues, microvessels were isolated according to the procedure as previously described [21]. Then, the cells were cultured in an endothelium-specific medium until the endothelial cells migrated out of the microvessels and confluently spread across the entire Petri dish. The endothelial cells were further cultured in exosome-depleted FBS-containing (EXO-FBS-250 A-1; System Biosciences, Mountain View, CA, United States) medium for an additional 48 h. The supernatant was collected for extracting exosomes, and the exosomes thus obtained were considered exosomes secreted by endothelial cells. More than 106 endothelial cells were collected for extraction of total RNA. For the isolation and identification of exosomes, the cell medium was centrifuged at 2000 × g for 10 min, followed by centrifugation at 10,000 × g for 30 min. The supernatant was then centrifuged at 110,000 × g for 70 min at 4 °C and washed twice with PBS. The exosomes were resuspended in 100 μl of PBS. In the identification, exosomes were mixed with uranyl acetate in equal amounts and dropped onto a copper grid. Then, the morphology of the exosomes was observed by electron microscopy. The expression levels of the specific proteins TSG101, CD63, and Flotillin-1 in the isolated exosomes were verified by western blotting. The particle size and distribution of exosomes were determined by qNano. The endothelial cells were identified by immunocytochemistry as previously described [22].
RNA extraction
We first kept the exosome RNA extracted by the RNeasy Maxi kit (QIAGEN, GER) in RNase-free water. Then, we used a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA) to measure the RNA purity. The Qubit RNA Assay Kit in a Qubit 2.0 fluorometer (Life Technologies, CA, USA) was used to check the RNA concentration. The RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA) was used to assess the RNA integrity. Total RNA of endothelial cells was extracted using the mirVana miRNA Isolation Kit (Ambion) following the manufacturer’s protocol. RNA integrity was evaluated using the Agilent 2100 Bioanalyzer, as mentioned above. The samples with RNA integrity number (RIN) ≥7 were subjected to the subsequent analysis.
Preparation of the sequencing library and small RNA and mRNA sequencing
TruSeq Small RNA Sample Prep Kits (Illumina, San Diego, CA, USA) were used to generate the sequencing libraries according to the manufacturer’s protocol. The index codes were also added to attribute sequences to each sample. DNA fragments corresponding to 140–160 bp were recovered and dissolved in 8 μl of elution buffer. Finally, we used DNA High Sensitivity Chips to evaluate the library quality on the Agilent Bioanalyzer 2100 system. After clustering the index-coded samples, the cDNA library was sequenced on an Illumina HiSeq X Ten sequencing platform with 50-bp-long single-end reads generated. For mRNA-seq, the libraries were constructed using the TruSeq Stranded mRNA LTSample Prep Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Then, these libraries were sequenced on the Illumina HiSeq X Ten sequencing platform, and 150-bp paired-end reads were generated.
Processing and assembly of sequencing raw data
For miRNA-Seq, low-quality reads were filtered, and the reads with 5′ primer contaminants and poly (A) were removed. The reads without the 3′ adapter and insert tag, as well as the reads shorter than 15 nt and longer than 41 nt were filtered from the raw data, and then the clean reads were obtained. The clean reads were aligned and then subjected to the BLAST [23] search against Rfam v.10.1 (http://www.sanger.ac.uk/software/Rfam) [24] and GenBank databases (http://www.ncbi.nlm.nih.gov/genbank/). The known miRNAs were identified by aligning against the miRBase v.21 database (http://www.mirbase.org/) [25], and the known miRNA expression patterns in different samples were identified. Unannotated small RNAs were used to predict novel miRNAs via mirdeep2 [26]. Based on the hairpin structure of a pre-miRNA and the miRBase database, the corresponding miRNA star sequence was also identified. For mRNA-Seq, raw reads were processed using Trimmomatic [27]. The reads containing poly-N and the low-quality reads were removed to obtain the clean reads. Afterwards, the clean reads were mapped to the reference genome using hisat2 [28].
Differentially expressed gene analysis
miRNAs were quantified by transcripts per million (TPM), which was calculated via the formula below: Normalized expression = unique mapped read count / total reads × 1,000,000. The DESeq2 R package was used to identify the differentially expressed miRNAs between SHRs and WKY rats [29]. Significant differentially expressed miRNAs were defined with a p value <0.05 and a fold change >2 as the threshold. The target gene of a miRNA was predicted by miRanda software [30]. For mRNA, the FPKM [31] value of each gene was calculated using cufflinks [32], and the read counts of each gene were obtained by the htseq-count [33]. Differentially expressed genes were identified using the DESeq [29] package in R with the cutoff of p-value <0.05 and fold change >2, which is the same as that for miRNA.
Gene functions and pathway enrichment analysis
The clusterProfiler [34] package in R was employed to perform gene functions and pathway enrichment analysis using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway databases. GO (http://www.geneontology.org/) is a commonly used method for annotating genes and gene products with functions including molecular function (MF), biological pathways, and cellular component (CC) [35]. KEGG (https://www.genome.jp/kegg/) is a useful resource for systematic analysis of gene functions and related high-level genome functional information [36]. GO enrichment analysis was conducted to better understand the biological functions of the unique target genes that were also differentially expressed by abnormal miRNAs between SHRs and WKY rats. Based on the KEGG database [36], we also used KEGG enrichment analysis to identify the significant pathway of those genes we were interested in. Fisher’s exact test was employed to choose the significant pathway, and the cutoff of significance was based on the p-value and BH adjusted p-value.
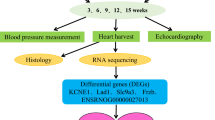
The experimental procedure of the entire study is detailed in Fig. 1.
Results
Characterization of endothelial cells and endothelial exosomes
Expression of the von Willebrand factor (vWF), as determined by immunofluorescence, was performed in isolated cells to identify them as endothelial cells (Fig. 2a). Exosomes isolated from the brain microvascular endothelial cells of SHRs and WKY rats were morphologically confirmed by transmission electron microscopy (TEM) (Fig. 2b). The diameter and size distribution of exosomes were further analyzed using nanoparticle tracking analysis (NTA) (Fig. 2c). Most of the particle diameters were distributed from 100 nm to 200 nm in width. Two exosome markers, namely, ALIX and TSG101, were clearly detected by western blot, further confirming the isolated exosomes (Fig. 2d).
Characterization of endothelial cells and endothelial exosomes. a The identification of endothelial cells by vWF (red) and DAPI (blue) (200×). b Exosomes were morphologically confirmed by TEM. c The diameter and size distribution of exosomes were analyzed using NTA. d Two exosome markers, namely, ALIX and TSG101, were clearly detected by western blot
Small RNA characterizations of SHRs and WKY rats
Exosomes contain various types of small RNAs, such as miRNA, rRNA, tRNA, snRNA, etc. The small RNAs were classified by mapping the unique reads to noncoding RNA databases such as the miRBase, RepeatMasker, and Rfam databases. We found that the majority of small RNA (~60–70%) from brain endothelial exosomes from SHRs and WKY rats was miRNA, followed by unknown small RNA types, which accounted for ~24–30% of all small RNAs (Fig. 3 and Table 1). Furthermore, we identified 159 novel miRNAs in total from the unannotated reads (Supplementary Table 1), and the known and novel miRNAs in different samples are shown in Table 2.
Differentially expressed gene analysis
Known and novel miRNAs identified were used for the identification of differentially expressed miRNAs. We identified 76 differentially expressed miRNAs in total, of which 37 were upregulated while 39 were downregulated in SHRs when compared to those in WKY rats (Fig. 4a). For the mRNAs, 1709 differentially expressed mRNAs in total were identified, with 775 upregulated and 934 downregulated (Fig. 4b).
miRNA target gene identification
To investigate the influence of miRNAs from the brain endothelial exosomes, we identified the target genes of the 76 differentially expressed miRNAs using miRanda. Of the 76 miRNAs identified, 60 (79%) miRNAs were predicted with target genes, and 6175 target genes were found in total (Fig. 5a). To further study the differentially expressed miRNAs, we focused only on the target genes that were also differentially expressed at the mRNA expression level between SHRs and WKY rats. These genes affected by differentially expressed miRNAs could play an important role in hypertension. By overlapping the target genes of 76 miRNAs and the 1709 differentially expressed mRNAs, we finally gained 647 genes targeted by 36 miRNAs (Fig. 5b and Supplementary Table 2), which were used for further investigation.
Gene function enrichment analysis of miRNA target genes
GO enrichment analysis was performed using the 647 target genes of 36 miRNAs that were differentially expressed in SHRs when compared to expression in WKY rats (Fig. 6). The results show that the target genes are mainly enriched in biological processes (BP), such as axon development, angiogenesis, and axonogenesis. As for the CC, they are significantly enriched in neuron-to-neuron synapses, extracellular matrix (ECM), cell–cell junctions, adherens junctions, etc. In addition, MF covers cell adhesion molecule (CAM) binding, growth factor binding, ECM binding, etc.
KEGG pathway enrichment analysis of miRNA target genes
KEGG pathway enrichment analysis was also conducted for the 647 target genes of 36 miRNAs that were differentially expressed in SHRs when compared to expression in WKY rats (Fig. 7). The results indicate that those genes were significantly enriched in axon guidance, focal adhesion, Cushing syndrome, CAMs, adherens junction, ECM-receptor interaction, and so on.
Discussion
Hypertension has a detrimental impact on target organs, resulting in end-stage renal disorders and serious cardiovascular diseases [1]. It is of great importance to uncover biomarkers or targets in the diagnosis and management of hypertension [37]. Endothelial cells, which exert an important role in modulating some arterial properties, including vascular permeability and vascular tone, regulate arterial stiffness via endothelial-derived substances [3]. Endothelial dysfunction is independently and strongly associated with hypertension [4].
miRNA is a small endogenous noncoding RNA that functions in RNA silencing and regulates gene expression at the posttranscriptional level [12]. Its deregulation could lead to metastasis and therapy resistance [38]. Exosomes produced in the endosomal compartment of most eukaryotic cells can travel throughout the body [14]. They contain a specific cargo of lipids, proteins, and miRNAs [15], which can regulate recipient cell behaviors and could be used as biomarkers for various human diseases. Increasing evidence indicates that the exosomal miRNAs are promising biomarkers in clinical diagnosis [39, 40]. Here, by studying the expression profiles of small RNAs of exosomes and mRNAs from brain endothelial cells in SHRs and WKY rats, we illustrated the critical roles of miRNAs in endothelial exosomes during hypertension development in the mammalian system in vivo.
Our findings revealed that miRNAs account for the majority (~60–70%) of small RNAs. More importantly, we identified 159 novel miRNAs in total from the unannotated reads across the diverse samples. A total of 76 differentially expressed miRNAs (37 upregulated, 39 downregulated) and 1709 differentially expressed mRNAs (775 upregulated, 934 downregulated) were found between SHRs and WKY rats. By identifying the target genes of those miRNAs, 647 genes targeted by 36 miRNAs have attracted further attention. These aberrant miRNAs and the genes changed and induced by abnormal miRNA expression may be potential biomarkers or therapeutic targets for hypertension. For example, miR-145, which is well known for its critical roles in the development of hypertension, is upregulated in exosomes derived from brain endothelial cells in SHRs when compared to levels in WKY rats. Importantly, consistent with a previous study, miR-145 has been reported to play important roles in the development of multiple cardiovascular diseases [41]. These data together support that these abnormal miRNAs identified above are potential biomarkers or therapeutic targets for hypertension.
Furthermore, it should be noted that pericytes could intercommunicate with endothelial cells via direct physical contact or paracrine signals [5, 10, 11], and exosomes can also fuse with other cells and transfer the miRNAs to the acceptor cell [17]. Zagrean et al. [42] described the multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia, which could be used for therapeutic implications. As a consequence, the roles of the abnormal miRNAs in the crosstalk between endothelial cells and pericytes are quite valuable for further investigation.
GO and KEGG enrichment analyses showed that the 647 target genes of 36 miRNAs were mainly enriched in axon development, angiogenesis, axonogenesis, neuron-to-neuron synapse, ECM, cell–cell junction, adherens junction, CAM binding, growth factor binding, and ECM-binding GO terms; as well as axon guidance, focal adhesion, Cushing syndrome, CAMs, adherens junction, and ECM-receptor interaction KEGG pathways. These GO terms and KEGG pathways were closely associated with hypertension, which could elucidate the mechanism of hypertension from miRNA regulation.
In summary, our study revealed miRNA changes and identified miRNA regulation mechanisms during mammalian hypertension development. We discovered that miRNA changes in hypertension cause several important gene function alterations and pathway dysregulation by regulating the gene expression of their target genes.
References
Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmcol Ther. 2006;111:81–98.
Serne EH, de Jongh RT, Eringa EC, RG IJ, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–11.
Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–6.
Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9.
Yuan X, Wu Q, Liu X, Zhang H, Xiu R. Transcriptomic profile analysis of brain microvascular pericytes in spontaneously hypertensive rats by RNA-Seq. Am J Transl Res. 2018;10:2372–86.
Pietri P, Vyssoulis G, Vlachopoulos C, Zervoudaki A, Gialernios T, Aznaouridis K, et al. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24:2231–8.
Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6:660–7.
Booth AD, Wallace S, McEniery CM, Yasmin, Brown J, Jayne DR, et al. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum. 2004;50:581–8.
Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–6.
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–13.
Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163–74.
Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep. 2017;7:5998.
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7:14293.
Alipoor SD, Tabarsi P, Varahram M, Movassaghi M, Dizaji MK, Folkerts G, et al. Serum exosomal miRNAs are associated with active pulmonary tuberculosis. Dis Markers. 2019;2019:1907426.
Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112:532–8.
Hornick NI, Huan J, Doron B, Goloviznina NA, Lapidus J, Chang BH, et al. Serum exosome microRNA as a minimally-invasive early biomarker of AML. Sci Rep. 2015;5:11295.
Bhome R, Del Vecchio F, Lee GH, Bullock MD, Primrose JN, Sayan AE, et al. Exosomal microRNAs (exomiRs): small molecules with a big role in cancer. Cancer Lett. 2018;420:228–35.
van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. S3991-3915.
Bovy N, Blomme B, Freres P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6:10253–66.
Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24.
Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–94.
Wu Q, Jing Y, Yuan X, Li B, Wang B, Liu M, et al. The distinct abilities of tube-formation and migration between brain and spinal cord microvascular pericytes in rats. Clin Hemorheol Microcirc. 2015;60:231–40.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res. 2003;31:439–41.
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–8.
Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1.
Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5.
Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
The Gene Ontology C. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017;45:D331–8.
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–61.
Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, et al. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS ONE. 2011;6:e21817.
Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90.
Liu X, Fortin K, Mourelatos Z. MicroRNAs: biogenesis and molecular functions. Brain Pathol. 2008;18:113–21.
Liu X, Yuan W, Yang L, Li J, Cai J. miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res. 2019;12:75–83.
Santovito D, Mandolini C, Marcantonio P, De Nardis V, Bucci M, Paganelli C, et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin Ther Targets. 2013;17:217–23.
Zagrean AM, Hermann DM, Opris I, Zagrean L, Popa-Wagner A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic implications. Front Neurosci. 2018;12:811.
Acknowledgements
This study was supported by the innovation fund of the Chinese Academy of Medical Sciences and Peking Union Medical College (Nos. 3332014006 and 3332015123), the CAMS Initiative for Innovative Medicine (CAMS-I2M) (No. 2016-I2M-3-006), and the National Natural Science Foundation of China (81801433).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, Q., Yuan, X., Li, B. et al. Integrated exosomal miRNA and transcriptome analysis of brain microvascular endothelial cells in spontaneously hypertensive rats. Hypertens Res 43, 90–98 (2020). https://doi.org/10.1038/s41440-019-0345-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-019-0345-0
Keywords
This article is cited by
-
Circulating exosomal MicroRNAs: New non-invasive biomarkers of non-communicable disease
Molecular Biology Reports (2021)