Abstract
This study investigated the association between maternal home blood pressure (HBP) trajectory during pregnancy and infant birth weight. A total of 755 pregnant women were included in this prospective cohort study. A group-based trajectory model identified six trajectory groups for home systolic blood pressure (SBP), diastolic BP (DBP), and mean arterial pressure (MAP). Next, the association of HBP trajectory groups with infant birth weight was evaluated using a general linear model considering potential confounding factors. For home SBP and MAP, the trajectory groups with a low-steep J-curve, moderate J-curve, little high J-curve, and high J-curve were significantly associated with lower infant birth weight than the low-J-curve group. Among the trajectory groups for home DBP, the moderate–steep J-curve, little high J-curve, and high J-curve were significantly associated with lower infant birth weight than the group with low-J-curve. The effect sizes of the trajectory groups varied in infant birth weight from −0.21 standard deviations (SDs) (95% confidence interval (CI): −0.42 to −0.01 SD) to −1.13 SD (95% CI: −1.54 to −0.72 SD). In the analyses of infant birth weight in grams, effect sizes that were significantly associated with infant birth weight varied from −84 g (95% CI: −167 to −1 g) to −567 g (95% CI: −732 to −402 g). Trajectory groups with a moderate–reverse J-curve for home SBP, DBP, and MAP were not significantly associated with infant birth weight. Maternal HBP trajectory during pregnancy was an indicator of infant birth weight. Further studies evaluating the associations between HBP during pregnancy and other perinatal outcomes are needed.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP), a common perinatal complication, are a risk factor for adverse perinatal outcomes due to inadequate uteroplacental perfusion [1, 2]. In addition, HDP are also a risk factor for future maternal diseases, including cardiovascular disease, coronary heart disease, heart failure, and stroke [3]. Therefore, the evaluation of maternal blood pressure (BP) during pregnancy is essential in prenatal management. In principle, HDP are diagnosed based on clinic blood pressure (CBP) measurements assessed within the medical environment [4, 5]. Several studies have reported a J-curve change in CBP values during pregnancy [6, 7]. Furthermore, a change in CBP values during pregnancy is an indicator of perinatal outcomes, including infant birth weight and length of gestation [8,9,10].
For the general population, several guidelines recommend home blood pressure (HBP) measurements, as an out-of-office measurement approach, in addition to CBP measurements, to diagnose and classify hypertension as follows: hypertension, white-coat hypertension, and masked hypertension [5, 11]. Compared with CBP, HBP has shown strong predictive power for cardiovascular diseases and target organ damage [12].
In addition, several guidelines in the field of obstetrics suggest performing HBP measurements in addition to CBP measurements during pregnancy [4, 13,14,15]. However, investigations evaluating the association between maternal HBP values during pregnancy and perinatal outcomes are limited [16]. Infant birth weight, a perinatal outcome, is inversely associated with coronary heart disease, type 2 diabetes mellitus (DM), and end-stage renal diseases later in life [17,18,19]. Since infant birth weight has decreased and the frequency of low birth weight infants has grown in Japan since 1980, the potential for an increased incidence of DM and hypertension has been underlined [20, 21]. Although maternal HBP values change during pregnancy similarly to the J-curve change in CBP values, the association of HBP trajectories with infant birth weight is unknown [22, 23]. Therefore, this study was conducted to investigate the association between maternal HBP trajectories during pregnancy and infant birth weight.
Methods
Study design and participants
This study was part of the Babies and their parents’ longitudinal Observation in Suzuki memorial Hospital on Intrauterine period (BOSHI) study, a prospective cohort study. The BOSHI study was conducted at the Suzuki Memorial Hospital, an obstetrical and gynecological hospital in the Sendai City area, Miyagi prefecture, Japan [24,25,26,27,28]. The Institutional Review Board of the Tohoku University School of Medicine and the Hospital Review Board of Suzuki Memorial Hospital approved the study protocol. Details of the BOSHI study have been described previously [24, 26].
A total of 4278 women were diagnosed with an intrauterine pregnancy between October 16, 2006 and July 31, 2010 at the Suzuki Memorial Hospital. Of 1611 women who were informed of the BOSHI study, 1066 women consented to participate. Women who consented after delivery (n = 178) or those who experienced twin pregnancies (n = 8) were excluded, and the remaining 880 women were included in this study.
Home blood pressure measurement during pregnancy
Physicians, midwives, or pharmacists instructed subjects on how to measure HBP after enrollment. HBP was measured according to the Japanese Society of Hypertension guidelines for self-monitoring of BP at home [29]. Subjects were asked to measure their HBP on their upper arm every morning, within 1 h of waking, after micturition, before breakfast, while seated, and after resting for at least 1 min [24, 26].
HBP was measured using an HEM-747IC or HEM-7080IC (Omron Health care, Kyoto, Japan), a semiautomatic device based on the cuff-oscillometric method and equipped with a digital display system [24, 26]. These devices used cuffs, and the algorithms were equivalent to the Omron T9P, which has been validated in pregnant women [30]. After the HBP values were entered on the survey forms, the values were entered into the database. The mean arterial pressure (MAP) was calculated based on the following formula: (SBP − DBP)/3 + DBP. The number of HBP measurements achieved at <10 weeks of gestation or at ≥41 weeks of gestation was too small to be analyzed in this study. Therefore, we used the average home SBP, DBP, and MAP values for each 1-week interval measured between the period of 10 weeks 0 days and 40 weeks 6 days.
Infant birth weight
Infant birth weight in grams was collected from the medical records. Information on the delivery week, parity, and infant sex was also collected from medical records to calculate the standard deviation (SD) value for infant birth weight because the reference values for Japanese infant birth weights are based on delivery week, parity, infant sex, and birth weight [31].
Other variables used in this study
Maternal age at the time of consent to research participation, body weight at the last prenatal checkup before delivery, information about assisted reproductive technology (ART), history of HDP, and the expected date of confinement (EDC) were collected from medical records. The EDC was confirmed by fetal crown-rump length as measured by ultrasonography at <12 weeks of gestation. The season of the EDC was defined as follows: spring (March, April, and May), summer (June, July, and August), autumn (September, October, and November), and winter (December, January, and February). Maternal height, prepregnancy body weight, family history of hypertension, smoking status, and alcohol consumption data were also collected with questionnaires and surveys by midwives. Smoking status was classified as follows: no smoking before conception, smoking until conception was recognized, and smoking during pregnancy. Alcohol consumption was classified as follows: no alcohol intake before conception, alcohol intake until conception was recognized, and alcohol intake during pregnancy. The prepregnancy body mass index (BMI) was calculated as follows: prepregnancy body weight in kilograms/(height in meters) [2]. Gestational weight gain was calculated as follows: body weight at the last prenatal checkup before delivery − prepregnancy body weight. Maternal diseases, including chronic hypertension, hyperthyroidism, hypothyroidism, antiphospholipid syndrome, and immunoglobulin A nephropathy, and obstetric complications, including HDP, placental abruption, and gestational DM (GDM), were also obtained from medical records.
In principle, HDP were diagnosed based on the CBP value [32]. In Japan, CBP is measured at prenatal checkups, which are conducted every 4 weeks at <23 weeks of gestation, once every 2 weeks at 23–35 weeks of gestation, and once per week at ≥36 weeks of gestation [24]. The method of measuring CBP in this study has been described previously [26].
Statistical analyses
First, we applied a group-based trajectory model (GBTM) to explore maternal HBP trajectory groups during pregnancy [33]. The quadratic order of the polynomial functions of the gestational week in each HBP trajectory group used was based on that from previous studies [22, 24, 34]. Next, we determined the number of HBP trajectory groups with each HBP trajectory function expressed using both a point estimate and 95% confidence interval. Details of the analysis using a GBTM are described in Supplementary Information.
We then explored maternal factors related to the HBP trajectory group assignment using a multinomial logistic regression model. Finally, we investigated the association between HBP trajectory groups and infant birth weight using a general linear model. HBP trajectory groups, as the exposures, and infant birth weight in SD value or in grams, as the outcome, were included in the model. Model 1 was a crude analysis. In model 2, infant birth weight in grams was regressed on the trajectory groups and potential confounding factors, including maternal age at the time of consent to research participation, height, prepregnancy BMI, parity, gestational weight gain, ART, family history of hypertension, smoking status, alcohol consumption, history of HDP, hyperthyroidism, hypothyroidism, season of expected date confinement, and infant sex. In model 2 for infant birth weight as an SD score, parity, delivery week, and infant sex were not included because the SD value of infant birth weight was calculated based on parity, delivery week, and infant sex in Japan [31]. Maternal age, height, prepregnancy BMI, and gestational weight gain were included as continuous variables in the model. HDP was not included in the model as an independent variable because it would become an intermediate variable. As an additional analysis, model 3 included average home SBP, DBP, or MAP at 10–15 weeks of gestation as the baseline HBP, in addition to the variables in model 2. Because the average home SBP, DBP, or MAP at 10–15 weeks of gestation had missing data, we applied multiple imputation. As the missing pattern was monotone, we used a regression imputation method. We constructed an imputation model using both independent variables and dependent variables in model 2 and HDP as a potential auxiliary variable. After 30 data sets were created, the same analysis was conducted for each data set. Next, the results of each analysis were combined using Rubin’s rule [35].
A two-sided p value < 0.05 was considered statistically significant. We used SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) to perform all statistical analyses.
Results
Maternal characteristics of the study participants
Figure 1 shows the flow chart of this study. Among the 880 women enrolled, women who withdrew consent (n = 3), underwent an abortion (n = 6), experienced intrauterine fetal death with fetal hydrops and congenital heart malformation (n = 1), delivered at other hospitals (n = 19), delivered at ≥42 weeks of gestation (n = 5) (due to lack of reference of Japanese infant birth weight at ≥42 weeks of gestation), and had missing HBP data during pregnancy (n = 91) were excluded from the study. Finally, 755 pregnant women were analyzed in this study.
Table 1 shows the maternal and neonatal characteristics of the analyzed subjects. The number and percentage of subjects who had HDP were 91% and 12.1%, respectively. The mean (SD) of infant birth weight in grams and those in SD values were 3062 (403) g and 0.1 (1.0), respectively.
Trajectory groups for HBP during pregnancy
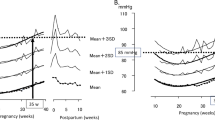
Six trajectory groups each for home SBP, DBP, and MAP measurements during pregnancy were identified independently using a GBTM (Fig. 2). As shown in Supplementary Table 1, all models satisfied the diagnostic criteria of model accuracy described in the statistical analysis section in Supplementary Information [33].
Figure 2 shows the estimated functions of the home SBP, DBP, and MAP trajectory groups during pregnancy. Trajectory groups included group 1 (low-J-curve), group 2 (low-steep J-curve), group 4 (moderate–reverse J-curve), group 5 (little high J-curve), and group 6 (high J-curve) for all the home SBP, DBP, and MAP measurements. Home SBP and MAP trajectory group 3 and home DBP trajectory group 3 were called moderate J-curve and moderate–steep J-curve, respectively. Almost all home SBP and DBP trajectory groups were <135/85 mmHg except for group 6 for home DBP.
Among the home SBP trajectory groups, group 2 (low-steep J-curve) had the greatest increase in SBP at ≥20 weeks of gestation. In contrast, the SBP in group 4 (moderate–reverse J-curve) decreased from 10 to 30 weeks of gestation and then slightly increased. Other trajectory groups showed similar shapes to each other, although the SBP values at 10 weeks of gestation differed. For the home DBP trajectory groups, group 3 (moderate–steep J-curve) showed the greatest increase in DBP at ≥20 weeks of gestation. Group 4 (moderate–reverse J-curve) showed a decrease in DBP from 10 to ~30 weeks of gestation and then DBP increased slightly. Group 6 (high J-curve) had the greatest decrease in DBP from 10 to ~25 weeks of gestation, with a rise in DBP towards 40 weeks of gestation. The six home MAP trajectory groups had shapes similar to those of the home SBP trajectory groups.
Association between maternal characteristics and HBP trajectory group assignment during pregnancy
Supplementary Tables 2–4 (supplementary information) show the differences in maternal and neonatal characteristics according to the home SBP, DBP, and MAP trajectory groups, respectively. Supplementary Tables 5–7 show the adjusted odds ratios of maternal factors for the home SBP, DBP, and MAP trajectory groups, respectively. Prepregnancy BMI, gestational weight gain, family history of hypertension, and season of EDC were significantly related to the home SBP, DBP, and MAP trajectory group assignments. Maternal height and smoking during pregnancy were associated with the home SBP trajectory group assignment. Associations between parity and home SBP, DBP, and MAP trajectory groups were not statistically significant.
Association between HBP trajectory groups during pregnancy and infant birth weight
Tables 2 and 3 show the results of the association between HBP trajectory groups and infant birth weight as SD values and grams, respectively.
In model 2, relative to home SBP, women who belonged to groups 2 (low-steep J-curve), 3 (moderate J-curve), 5 (little high J-curve), and 6 (high J-curve) delivered significantly lower infant birth weight infants compared with those who belonged to group 1 (low-J-curve). In model 2, relative to home DBP, women who belonged to groups 3 (moderate–steep J-curve), 5 (little high J-curve), and 6 (high J-curve) delivered significantly lower infant birth weight infants compared with those who belonged to group 1 (low-J-curve). In model 2, relative to home MAP, women who belonged to groups 2 (low-steep J-curve), 3 (moderate J-curve), 5 (little high J-curve), and 6 (high J-curve) delivered significantly lower infant birth weight infants compared with those who belonged to group 1 (low-J-curve), with the exception of the association between group 2 (low-steep J-curve) and infant birth weight measured as an SD value. For home SBP, DBP, and MAP, group 4 (moderate–reverse J-curve) was not significantly associated with infant birth weight.
In model 3, almost all associations between HBP trajectory groups and infant birth weight were similar, although the effect sizes were slightly weakened compared with those of model 2.
Discussion
To the best of our knowledge, this study showed for the first time that maternal HBP trajectory during pregnancy was an indicator of infant birth weight. HBP trajectory during pregnancy may provide health care providers information that reflects perinatal outcome. Previous studies have reported an inverse linear association between infant birth weight and adult hypertension and adult-onset DM in Japan [36,37,38]. Katanoda et al. reported that a 100 g increase in birth weight had significantly lower odds of adult-onset DM in Japan. Conversely, a 100 g decrease in birth weight had higher odds of adult-onset DM [38]. Almost all effect sizes derived from the HBP trajectory groups which were statistically significantly associated with lower infant birth weight, were decreases of 100 g or more. Therefore, the impact of maternal HBP trajectory to effect sizes in infant birth weight in this study would be clinically meaningful in Japan. Even after adjustment for HBP at 10–15 weeks of gestation, HBP trajectory was an indicator of infant birth weight. The higher number of BP measurements used for the trajectory model, compared with the number of BP measurements in early pregnancy, might have contributed to the stronger association; additional studies might be necessary to validate this comparison.
In this study, subjects who had a family history of hypertension or those whose season of EDC was spring or winter had significantly higher odds of belonging to the HBP trajectory groups that were significantly associated with lower infant birth weight. Smoking during pregnancy also had significantly higher odds of assignment to a home SBP trajectory group that was significantly associated with lower infant birth weight. Therefore, family history of hypertension, season of EDC, and smoking status during pregnancy may represent important indicators of HBP trajectory during pregnancy.
The findings of this study were consistent with those of a previous study indicating that HBP during pregnancy was associated with perinatal outcome. Inoue et al. reported that a high HBP value at <20 weeks of gestation was a risk factor for HDP, diagnosed based on CBP values at ≥20 weeks of gestation [39].
The percentage of HDP in HBP trajectory groups with significantly lower infant birth weight infants was higher than that in the groups that were not statistically associated with infant birth weight in this study. Therefore, the HBP trajectory might reflect inadequate uteroplacental perfusion due to HDP.
Although studies supporting recommendations for the measurement of HBP in addition to CBP during pregnancy are limited, several guidelines have proposed a classification for HDP based on both CBP and HBP [4, 14]. In 2018, the International Society for the Study of Hypertension in Pregnancy suggested a classification for HDP using both CBP and HBP values [4]. At <20 weeks of gestation, chronic hypertension is diagnosed if CBP is ≥140/90 mmHg and HBP is ≥135/85 mmHg. White-coat hypertension is diagnosed with CBP ≥ 140/90 mmHg and HBP < 135/85 mmHg. Masked hypertension is also diagnosed if CBP is <140/90 mmHg and HBP is ≥135/85 mmHg [4]. The French Society of Hypertension also indicated that HBP ≥ 135/85 mmHg during pregnancy is considered pathological [40]. These diagnostic criteria are derived from thresholds used in the general population [5]. However, HBP ≥ 135/85 mmHg as a threshold for diagnosis of HDP regardless of gestational age may not be appropriate as HBP changes during pregnancy. Mikami et al. proposed provisional criteria for diagnosis of HDP according to gestational age using HBP [23]. In their study, HBP values equal to CBP 140/90 mmHg were 120.8/83.5 mmHg, 126.0/85.2 mmHg, and 136.3/89.3 mmHg in the first, second, and third trimesters, respectively [23]. In this study, several maternal HBP trajectory groups were significantly associated with lower infant birth weight even when home SBP and home DBP values throughout pregnancy were <135 mmHg and <85 mmHg, respectively. Further accumulation of evidence regarding the association between HBP during pregnancy and other perinatal outcomes, including placental abruption, fetal growth restriction, and fetal distress, is needed to determine the threshold of HBP for diagnosing HDP.
It should be noted that this study does not overlook the importance of CBP measurements, as findings relative to the association of CBP during pregnancy with perinatal outcomes have accumulated in previous studies [9, 10, 41,42,43]. Although several studies have reported that the number of prenatal visits was reduced by HBP measurements during pregnancy, no previous studies have clarified whether HBP measurements, in addition to CBP measurements, improve perinatal outcomes [44, 45]. Therefore, further studies will need to examine whether HBP measurements in combination with CBP measurements during pregnancy would improve perinatal outcomes. In addition, pregnant women must buy devices for self-monitoring of HBP, except those who participate in clinical studies, if a health care provider in Japan recommends that a pregnant woman monitor HBP. Therefore, the costs and benefits of measuring HBP during pregnancy should be examined.
This study has several limitations. First, this study was conducted at a single hospital. Although external validity will likely support our results, as in our previous studies, the findings in this study need to be reproduced in other populations [26, 28]. Second, the association of the HBP trajectory group with other perinatal outcomes, including placental abruption and preterm delivery, could not be evaluated because the sample size was too small to be analyzed. Therefore, studies with a larger sample size will be needed. Third, differences in angiogenic imbalances among HBP trajectory groups could not be examined because neither soluble fms-like tyrosine kinase 1 nor placental growth factor levels were measured in this study. Furthermore, we could not assess whether subjects adequately measured BP at home, although physicians, midwives, or pharmacists individually instructed the subjects on how to measure HBP after enrollment.
In conclusion, maternal HBP trajectory during pregnancy was an indicator of infant birth weight. Thus, health care providers may consider HBP meaningful clinical information for pregnant women who monitor HBP values during pregnancy.
References
Brown CM, Garovic VD. Mechanisms and management of hypertension in pregnant women. Curr Hypertens Rep. 2011;13:338–46.
ACOG Practice Bulletin No. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25.
Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213–20.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;37:253–390.
Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Established preeclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children. J Hypertens. 2011;29:1703–11.
Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, et al. Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension. 2012;60:1250–6.
Iwasaki R, Ohkuchi A, Furuta I, Ojima T, Matsubara S, Sato I, et al. Relationship between blood pressure level in early pregnancy and subsequent changes in blood pressure during pregnancy. Acta Obstet Gynecol Scand. 2002;81:918–25.
Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension. 2014;64:36–44.
Wu Y, Ma Y, Wu K, Zhao W, Hu H, Yang Q, et al. Blood pressure in early and mid-pregnancy and the risk of small-for-gestational-age birth: findings of a large cohort study in China. J Hum Hypertens. 2019;33:475–81.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115.
Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15:413.
Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–31.
Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–45.
Stepan H, Kuse-Fohl S, Klockenbusch W, Rath W, Schauf B, Walther T, et al. Diagnosis and treatment of hypertensive pregnancy disorders. Guideline of DGGG (S1-Level, AWMF Registry No. 015/018, December 2013). Geburtshilfe Frauenheilkd. 2015;75:900–14.
Bello NA, Miller E, Cleary K, Wapner R, Shimbo D, Tita AT. Out of office blood pressure measurement in pregnancy and the postpartum period. Curr Hypertens Rep. 2018;20:101.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7.
Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97.
Ruggajo P, Skrunes R, Svarstad E, Skjaerven R, Reisaether AV, Vikse BE. Familial factors, low birth weight, and development of ESRD: a Nationwide Registry Study. Am J Kidney Dis. 2016;67:601–8.
Gluckman PD, Seng CY, Fukuoka H, Beedle AS, Hanson MA. Low birth weight and subsequent obesity in Japan. Lancet. 2007;369:1081–2.
Normile D. Staying slim during pregnancy carries a price. Science. 2018;361:440.
Denolle T, Daniel JC, Calvez C, Ottavioli JN, Esnault V, Herpin D. Home blood pressure during normal pregnancy. Am J Hypertens. 2005;18:1178–80.
Mikami Y, Takai Y, Era S, Ono Y, Saitoh M, Baba K, et al. Provisional criteria for the diagnosis of hypertension in pregnancy using home blood pressure measurements. Hypertens Res. 2017;40:679–84.
Metoki H, Ohkubo T, Watanabe Y, Nishimura M, Sato Y, Kawaguchi M, et al. Seasonal trends of blood pressure during pregnancy in Japan: the babies and their parents’ longitudinal observation in Suzuki Memorial Hospital in Intrauterine Period study. J Hypertens. 2008;26:2406–13.
Metoki H, Ohkubo T, Obara T, Akutsu K, Yamamoto M, Ishikuro M, et al. Daily serial hemodynamic data during pregnancy and seasonal variation: the BOSHI study. Clin Exp Hypertens. 2012;34:290–6.
Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, et al. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens. 2013;26:141–8.
Ishikuro M, Obara T, Metoki H, Ohkubo T, Iwama N, Katagiri M, et al. Parity as a factor affecting the white-coat effect in pregnant women: the BOSHI study. Hypertens Res. 2015;38:770–5.
Iwama N, Metoki H, Ohkubo T, Ishikuro M, Obara T, Kikuya M, et al. Maternal clinic and home blood pressure measurements during pregnancy and infant birth weight: the BOSHI study. Hypertens Res. 2016;39:151–7.
Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H. et al. The Japanese Society of Hypertension Guidelines for self-monitoring of blood pressure at home. Hypertens Res. 2012;35:777–95.
Brown MA, Roberts L, Davis G, Mangos G. Can we use the Omron T9P automated blood pressure monitor in pregnancy? Hypertens Pregnancy. 2011;30:188–93.
Itabashi K, Fujimura M, Kusuda S, Tamura M, Hayashi T, Takahashi T, et al. New normative birth weight among Japanese infants according to gestational week at delivery. Acta Paediatr Jpn. 2010;114:1271–93. (In Japanese)
Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, et al. New definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”. Hypertens Res Pregnancy. 2017;5:39–40.
Nagin DS. Group-based modeling of development. Harvard University Press; Cambridge, MA. 2005.
Silva LM, Steegers EA, Burdorf A, Jaddoe VW, Arends LR, Hofman A, et al. No midpregnancy fall in diastolic blood pressure in women with a low educational level: the Generation R Study. Hypertension. 2008;52:645–51.
Michael O, Bohdana R. Clinical Trials with Missing Data, A Guide for Practitioners. Chichester: John Wiley & Sons Ltd; 2014. p. 185–256. https://onlinelibrary.wiley.com/doi/book/110.1002/9781118762516
Tamakoshi K, Yatsuya H, Wada K, Matsushita K, Otsuka R, Yang PO, et al. Birth weight and adult hypertension: cross-sectional study in a Japanese workplace population. Circ J. 2006;70:262–7.
Oya J, Nakagami T, Kurita M, Yamamoto Y, Hasegawa Y, Tanaka Y, et al. Association of birth weight with diabetes and insulin sensitivity or secretion in the Japanese general population. J Diabetes Investig. 2015;6:430–5.
Katanoda K, Noda M, Goto A, Mizunuma H, Lee JS, Hayashi K. Impact of birth weight on adult-onset diabetes mellitus in relation to current body mass index: the Japan Nurses’ Health Study. J Epidemiol. 2017;27:428–34.
Inoue M, Tsuchihashi T, Hasuo Y, Ogawa M, Tominaga M, Arakawa K, et al. Salt intake, home blood pressure, and perinatal outcome in pregnant women. Circ J. 2016;80:2165–72.
Mounier-Vehier C, Amar J, Boivin JM, Denolle T, Fauvel JP, Plu-Bureau G, et al. Hypertension and pregnancy: expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Fundam Clin Pharm. 2017;31:83–103.
Bakker R, Steegers EA, Hofman A, Jaddoe VW. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the Generation R Study. Am J Epidemiol. 2011;174:797–806.
Wikstrom AK, Gunnarsdottir J, Nelander M, Simic M, Stephansson O, Cnattingius S. Prehypertension in pregnancy and risks of small for gestational age infant and stillbirth. Hypertension. 2016;67:640–6.
Zhu B, Huang K, Bao W, Yan S, Hao J, Zhu P, et al. Dose-response relationship between maternal blood pressure in pregnancy and risk of adverse birth outcomes: Ma’anshan birth cohort study. Pregnancy Hypertens. 2019;15:16–22.
Ross-McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, et al. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG. 2000;107:217–21.
Perry H, Sheehan E, Thilaganathan B, Khalil A. Home blood-pressure monitoring in a hypertensive pregnant population. Ultrasound Obstet Gynecol. 2018;51:524–30.
Acknowledgements
This study was supported by Grants for Scientific Research (18590587, 18390192, 21390201, 24689061, 25253059, 26860412, 16H05243, 17K15857, 18K15837, and 19H03905) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a Grant-in-Aid (19DA1001) for Health Research on Children, Youth and Families, and (H21-Junkankitou [Seishuu]-Ippan-004) from the Ministry of Health, Labor and Welfare, Health and Labor Sciences Research Grants, Japan; Grant (JP19gk0110039) from AMED; and a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) fellows (19.7152, 20.7198, 20.7477, and 20.54043). There were also Academic Contributions from Pfizer Japan Inc.; Bayer Academic Support; Takeda Research Support; Astellas Research Support; Scholarship donations from Chugai Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd.
Members of the BOSHI Study Group
Medical members (Obstetrics): Noriyuki Iwama, Hidekazu Nishigori, Kohei Tanaka, Takashi Sugiyama, Junichi Sugawara, Nobuo Yaegashi, Kazuhiko Hoshi, Kunihiko Okamura, Masakuni Suzuki. Medical members (Internal Medicine): Hirohito Metoki, Kei Asayama, Ryusuke Inoue, Masahiro Kikuya, Takayoshi Ohkubo, Shinichi Kuriyama, Yutaka Imai. Coordinating members: Taku Obara, Mami Ishikuro, Rie Tsuchida, Azusa Hara, Takuo Hirose, Takeshi Kobayashi, Kenta Gonokami, Takanao Hashimoto, Yumiko Watanabe, Misato Nishimura, Maiko Kawaguchi, Yurie Sato, Minako Hoshikawa, Ayano Sasaki, Kasumi Sakurai, Michihiro Sato, Konomi Akutsu, Mami Yamamoto, Aya shiraishi, Miki Hosaka. Clinical examination members: Ikuo Tachibana, Maki Omura, Mikiko Ishikawa, Yoshimi Fujii, Hidemi Kobayashi, Kazuyuki Akaishi. Pharmaceutical members: Yuko Kikuchi, Kei Tate, Chieko Koishi, Saori Sugawara. Recruitment members: Katsuyo Yagihashi, Junko Saitou, Hiromi Sasaki, Tomoko Suzuki, Junko Takahashi, Yoko Narita, Satoko Shigihara, Hideko Tada, Yumi Hamada. Outpatient management members: Nozomi Satou. Nami Satou, Setsuko Sai, Nana Atsumi, Naoko Sekine, Yukari Ueno, Yu Itou. Inpatient management members: Yukie Obara, Nami Onodera, Asako Sato, Youko Iwasa, Mamiko Abe, Yukari Kido, Risako Komuro, Yukiko Nakamura, Marie Watanabe, Chikako Matsumoto, Koto Oyama, Aya Takahashi, Michiko Kojima, Miyuki Abe, Mariko Sane, Mana Takahashi, Kana Sugata Miho Igari, Haruhi Sasaki, Mizuki Kobayashi, Aya Kikuchi, Risa Yamamoto, Akiho Goto, Eri Yamauchi, Mika Chiba, Sakiko Ota, Hiromi Ishikawa, Akemi Sasaki, Tomoko Kawamura, Hiroko Hiji, Misaki Kishinami, Yurie Kowata, Eiko Yamauchi, Yasuko Takahashi, Naho Sato.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
TO, YI, TO, and HM concurrently hold the directorship at the Tohoku Institute for Management of Blood Pressure, which is supported by Omron Health care Co. Ltd. HM is also currently involved in collaborative research with Omron Health care in another study. Omron Health care Co. Ltd. was not involved in the study design, paper methodology, writing, or drafting of the paper or its conclusions.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Iwama, N., Oba, M.S., Satoh, M. et al. Association of maternal home blood pressure trajectory during pregnancy with infant birth weight: the BOSHI study. Hypertens Res 43, 550–559 (2020). https://doi.org/10.1038/s41440-020-0416-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0416-2
Keywords
This article is cited by
-
Machine learning approaches for predicting fetal macrosomia at different stages of pregnancy: a retrospective study in China
BMC Pregnancy and Childbirth (2025)
-
Associations of prenatal blood pressure trajectory and variability with child neurodevelopment at 2 years old
BMC Medicine (2024)
-
Development and evaluation of machine learning models for predicting large-for-gestational-age newborns in women exposed to radiation prior to pregnancy
BMC Medical Informatics and Decision Making (2024)
-
Long-term changes in blood pressure and their health impact
Hypertension Research (2023)
-
A personal history of research on hypertension From an encounter with hypertension to the development of hypertension practice based on out-of-clinic blood pressure measurements
Hypertension Research (2022)