Abstract
Our objective was to examine whether high blood pressure in the preconception period was associated with gestational hypertension and preeclampsia in Chinese women. Data were obtained from the China-US Collaborative Project for Neural Tube Defects Prevention, a large population-based cohort study. We included 45,628 women who were registered before pregnancy in seven counties in South China. Blood pressure was measured during registration by trained health care workers, and other health-related information was recorded prospectively. We used logistic regression to evaluate the associations between preconception blood pressure and the risk of gestational hypertension and preeclampsia, adjusting for potential confounders. The prevalence of hypertension in the preconception study population was 4.57% (2083/45,628). The incidences of gestational hypertension and preeclampsia were 11.95% and 4.08%, respectively, in the hypertension group and 8.60% and 2.28%, respectively, in the nonhypertension group. Compared with the nonhypertension group, the hypertension group showed a significantly increased risk for gestational hypertension [adjusted risk ratio (RR) = 1.40, 95% confidence interval (CI): 1.22–1.60] and preeclampsia [adjusted RR = 1.75, 95% CI: 1.39–2.19]. When participants with normal blood pressure were used as the reference, the adjusted ORs for gestational hypertension were 1.48 (95% CI: 1.37–1.59), 1.70 (95% CI: 1.44–2.01), and 1.29 (95% CI: 1.02–1.64), and for preeclampsia, the adjusted ORs were 1.55 (95% CI: 1.35–1.78), 1.95 (95% CI: 1.46–2.60), and 1.99 (95% CI: 1.39–2.85) for the participants with prehypertension, stage 1 hypertension, and stage 2 hypertension, respectively. Our results support an association between hypertension or higher blood pressure prior to pregnancy and an increased risk of gestational hypertension and preeclampsia.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy are one of the most common complications, affecting 1–10% of pregnant women worldwide, especially in developing countries [1]. Gestational hypertension and preeclampsia are the most common clinical forms of hypertensive disorders of pregnancy. Among these conditions, preeclampsia involves not only elevations in blood pressure and proteinuria but also other systemic clinical manifestations. This multisystem syndrome is associated with the most significant immediate risk to mothers and their offspring’s cardiovascular morbidity and mortality [2, 3]. Gestational hypertension and preeclampsia usually occur due to the lack of or inadequacy of medical care during pregnancy or prepregnancy periods. Preexisting maternal and obstetric conditions are present in a high proportion of the population at risk for gestational hypertension and preeclampsia [4].
Gestational hypertension and preeclampsia have been associated with preconception blood pressure levels, especially when significant hypertension is present before pregnancy [5, 6]. Few studies, however, have investigated the association between preconception blood pressure and gestational hypertension and preeclampsia. From a preventive point of view, understanding the impact of preconception blood pressure is of particular interest because it is relatively easy to intervene before pregnancy, and this may have a greater impact on gestational outcome. Hence, we hypothesized that preconception blood pressure would be positively correlated with gestational hypertension and preeclampsia and investigated this hypothesis by conducting a large population study.
Pregnant women with gestational hypertension can eventually develop preeclampsia, so there was also a question regarding whether preconception hypertension could contribute to gestational hypertension without preeclampsia. Large cohorts are essential to assess the risk of those rare outcomes. Furthermore, gestational hypertension and preeclampsia may have important etiologic differences [7], but no study to date has compared the differences in preconception blood pressure levels between individuals with these outcomes. We investigated those questions to improve the understanding of the mechanism underlying each subtype. We used the data from a large prospective population-based cohort study to investigate whether a woman’s blood pressure just prior to pregnancy has any impact on the risk of gestational hypertension and preeclampsia. We also assessed whether the impact of preconception blood pressure on gestational hypertension and preeclampsia was the same.
Methods
Background and original cohort
The methods of the original study have been described previously [8, 9]. Beginning in 1993, the Chinese Ministry of Health conducted a public health campaign to prevent neural tube defects in 21 counties in two southern provinces (Zhejiang and Jiangsu) and one northern province (Hebei). During this campaign, all female residents of the included counties who were getting married or who became pregnant were registered in a pregnancy monitoring system that served as the principal record of prenatal care and the source of demographic information. All women were advised to take a pill solely containing 400 μg of folic acid every day, starting at the time of registration on the pregnancy monitoring system and continuing until completion of the first trimester of pregnancy. If women consented to take folic acid, the pills were distributed at the time of registration. At the end of each month, health workers recorded the dates of all menstrual periods and how many pills remained in each bottle (if the participant was taking pills). To evaluate the effect of folic acid on neural tube defects, we identified women who were registered in the monitoring system between October 1993 and September 1995, who delivered by 31 December 1996, and whose fetuses or infants could be confirmed as either having or not having a neural tube defect (whether live born, stillborn or electively terminated because of prenatal diagnosis of any birth defect). Miscarriages and elective terminations that took place before 20 weeks of gestation were not included in the cohort. The original cohort included a total of 247,831 women. The project was approved by the institutional review boards of the US Centers for Disease Control and Prevention and Peking University Health Science Center. All women who took pills provided oral informed consent.
Selection of study subjects
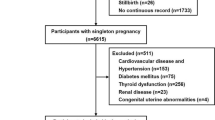
For this analysis, we selected seven counties in two neighboring southern provinces (Jiangsu Province and Zhejiang Province) that had detailed records of preconception examinations as well as gestational weeks, enabling us to examine the effect of preconception blood pressure and the risk of gestational hypertension and preeclampsia and to differentiate the effects according to gestational hypertension subtype. Of 86,057 women from the selected counties, we chose 46,525 women who registered in the health examination system before pregnancy as our target population. Of these women, we excluded 767 (1.65%) with missing systolic or diastolic blood pressure (SBP and DBP, respectively) data or outlying data (<60 and >220 for SBP, <40 and >140 for DBP) and 141 (0.30%) with unknown information about hypertensive disorders of pregnancy. After these exclusions, 45,628 participants (98.07% of the targeted population) were included in the final analysis. Information regarding the formation of the target recruitment population and derivation of the population used in the final analysis is shown in Fig. 1.
Blood pressure measurement and hypertension definition
Blood pressure was measured by trained heath care workers during registration. The appropriate cuff size was determined based on arm circumference. The measurement was performed on the right arm with a mercury sphygmomanometer and was performed on two or more consecutive occasions at an interval of 6 h.
Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. To investigate the associations between the various grades and types of hypertension and the development of gestational hypertension and preeclampsia, we further categorized blood pressure according to the 7th Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure as follows: (1) normal blood pressure (SBP < 120 mmHg and DBP < 80 mmHg); (2) prehypertension (SBP 120–139 mmHg or DBP 80–89 mmHg); (3) stage 1 hypertension (SBP 140–159 mmHg or DBP 90–99 mmHg); and (4) stage 2 hypertension (SBP ≥ 160 mmHg or DBP ≥ 100 mmHg) [10].
Based on the groupings of the World Health Organization and International Society of Hypertension Guidelines on Hypertension Management [11], participants were subsequently classified as follows: (1) normal blood pressure (SBP < 140 mmHg and DBP < 90 mmHg); (2) isolated diastolic hypertension (SBP < 140 mmHg and DBP ≥ 90 mmHg); (3) isolated systolic hypertension (SBP ≥ 140 mmHg and DBP < 90 mmHg); and (4) combined hypertension (SBP ≥ 140 mmHg and DBP ≥ 90 mmHg).
Definition of gestational hypertension and preeclampsia
Information on gestational hypertension and preeclampsia was abstracted from the pregnancy monitoring system that serves as the principal record of prenatal care and delivery. Gestational hypertension was defined as an absolute blood pressure ≥ 140/90 mmHg after 20 weeks of gestation or as a blood pressure increment of ≥30/15 mmHg after 20 weeks of gestation compared with the blood pressure during the first trimester. Chronic hypertension was defined as gestational hypertension and the maintenance of hypertension during pregnancy without blood pressure reductions in early pregnancy. Preeclampsia (including eclampsia) was defined as a blood pressure of ≥140/90 mmHg or a blood pressure increment of 30/15 mmHg after 20 weeks of gestation with concurrent proteinuria (a single random urine specimen containing at least 1+ protein by dipstick test) after 20 weeks of gestation [12]. We defined duration as the time between the baseline measurement and the index pregnancy.
Statistical analysis
We conducted an analysis of the mean age, body mass index (BMI) and distributions of parity, ethnic origin, education, and occupation between women who had or had not taken folic acid. The basic characteristics of pregnant women in the different study groups were compared using Student’s t test for quantitative variables and the chi-square test for categorical variables. We used logistic regression models to evaluate the risk ratios (RRs) of gestational hypertension and preeclampsia in the hypertension group, and we adjusted for maternal age (continuous), BMI (continuous), education, occupation, folic acid use, ethnicity, and parity (categorized as shown in Table 1). All data were analyzed using SPSS for Windows software (ver. 20.0; SPSS Inc., Chicago, IL, USA).
Results
Nearly all participants were of the Han ethnicity. Of the 45,628 total participants, 2083 (4.57%) were diagnosed with hypertension. The rate of prepregnancy hypertension and/or proteinuria was 8.6%. Table 1 lists the baseline characteristics of participants according to preconception hypertension status. Participants diagnosed with hypertension tended to be older, have a larger body size, be multipara and be more likely to be factory workers.
The total incidences of gestational hypertension and preeclampsia in our population were 8.75% (3992/45,628) and 2.36% (1078/45,628), respectively. The incidence of gestational hypertension was higher in the hypertension group (11.95%) than in the nonhypertension group (8.60%; Table 2). The adjusted RR showed a statistically significant increase in gestational hypertension risk in the hypertension group [adjusted RR = 1.40, 95% CI: 1.22–1.60]. Moreover, the incidence of preeclampsia was also higher in the hypertension group (4.08%) than in the nonhypertension group (2.28%) [adjusted RR = 1.75, 95% CI: 1.39–2.19].
The gestational hypertension and preeclampsia incidences and adjusted RRs across blood pressure categories are shown in Table 3. Using the normal BP category as a reference, the incidences of both gestational hypertension and preeclampsia significantly increased with the BP categories. Compared with the normal group, the multivariable-adjusted RRs of gestational hypertension were 1.48 (95% CI: 1.37–1.59), 1.70 (95% CI: 1.44–2.01), and 1.29 (95% CI: 1.02–1.64) for the women with prehypertension and stage 1 and stage 2 hypertension, respectively, whereas the RRs for preeclampsia were 1.55 (95% CI: 1.35–1.78), 1.95 (95% CI: 1.46–2.60), and 1.99 (95% CI: 1.39–2.85), respectively. When hypertension was divided into isolated systolic hypertension, isolated diastolic hypertension, and combined hypertension, women with isolated systolic hypertension [adjusted RR = 1.91, 95% CI: 1.38–2.64] and combined hypertension [adjusted RR = 1.74, 95% CI: 1.26–2.40] prior to pregnancy showed a significantly increased risk of preeclampsia. Only isolated systolic hypertension before pregnancy significantly increased the risk of gestational hypertension with a multivariable-adjusted RR of 1.69 (95% CI: 1.40–2.05). Significant and positive associations between the hypertension categories and gestational hypertension and preeclampsia incidences were identified (P values for linear trends < 0.01). Excluding major external birth defects from the analysis did not change our results (data not shown).
Discussion
In this large population-based cohort study, which included 45,628 pregnant women in China, we examined the relationship between preconception blood pressure and the risk of gestational hypertension and preeclampsia. Independent of maternal age, BMI, education, occupation, folic acid use, ethnicity and parity, our research revealed a significant association between maternal preconception blood pressure and gestational hypertension and preeclampsia risk. We also identified a significant relationship between different types of hypertension and gestational hypertension and preeclampsia.
Our interest in preconception blood pressure for the prediction of adverse events stems from the influence of blood pressure levels in the gestational period on adverse events. Many epidemiological studies have shown that excessive increases in maternal peripheral and central blood pressure during pregnancy were correlated with adverse pregnancy outcomes [13,14,15]. Some researchers have concluded that maternal hypertension prior to pregnancy can significantly increase the risk of preterm birth and low birth weight [16, 17]. Gestational hypertension and preeclampsia are among the most striking adverse pregnancy outcomes. Preeclampsia is the severe subtype of hypertensive disorders of pregnancy according to its clinical presentation. Differences in clinical features may be due to different mechanisms [18]. However, few studies have observed whether the association between pregestational BP and gestational hypertension is largely driven by one particular subtype over the others. Such a situation might weaken the association between pregestational BP and gestational hypertension overall. Moreover, an examination of associations within particular subtypes would reveal interesting clues into the cause and underlying biological mechanisms of gestational hypertension. Hence, we speculated that preconception high blood pressure would probably lead to gestational hypertension and preeclampsia.
Although many researchers have investigated the relationship between blood pressure changes during pregnancy periods and complications, few studies have investigated the effect of preconception blood pressure on gestational hypertension, especially according to gestational hypertension subtype. Egeland et al. [19] found that the estimated adjusted RRs for the associations between prepregnant hypertensive status and gestational hypertension and preeclampsia were 7.1 (4.84–10.44) and 3.5 (2.48–4.97), respectively. Another study involving HUNT-2 and Norway’s medical birth registry also indicated that women with systolic blood pressure levels in the highest quintile had a 7.3-times higher risk of developing preeclampsia than those with the lowest systolic blood pressure levels [20]. Our results are consistent with those findings. There was also an obvious significant dose-response relationship between preconception hypertension and gestational hypertension and preeclampsia. The associations of preconception blood pressure levels with gestational hypertension and preeclampsia were also reanalyzed after we excluded women who were hypertensive prior to pregnancy who maintained hypertension during pregnancy without a blood pressure decrease in early pregnancy. The results of the multivariable logistic analysis showed that compared with the pregestational nonhypertension group, the adjusted RRs of gestational hypertension and preeclampsia were 1.88 (1.40, 2.51) and 2.81 (1.83, 4.32), respectively. However, a Finnish study found that neither DBP nor SBP was significantly associated with gestational hypertension or preeclampsia [5]. These discrepancies require more research to draw accurate conclusions.
In our study, pregestational hypertension was linked to a greater than 40% increased risk of gestational hypertension and preeclampsia. Reduced maternal placental function is a possible mechanism underlying this result. Higher blood pressure before pregnancy is characterized by both endothelial dysfunction and decreased placental perfusion [21]. Uteroplacental vasculopathy, including histopathologic lesions and intravascular coagulation, is associated with gestational hypertension and preeclampsia through a two-stage process [22, 23]. These are all risk factors for altered prepregnancy hemodynamics and have been found to occur in women with preeclampsia [24].
Our study had several strengths. First, it used a population-based prospective cohort design, and data were collected on both exposure and outcome, thus minimizing the risk of selection and recall bias. Hypertension was diagnosed by measuring blood pressure directly, thus minimizing the likelihood of misclassification bias. In addition, we evaluated gestational hypertension and preeclampsia while excluding congenital anomalies and stillbirths, thus reducing information bias. The provinces of Jiangsu and Zhejiang are on the east coast of China, where the population is ethnically homogenous, with 99% being Han Chinese. Almost all women (99.4%) in our population were of Han ethnicity, i.e., their genetic background was homogeneous. The sample size was large enough to detect both overall and subgroup effects. Detailed data on pregnancy complications, as well as clinical records, allowed us to examine associations among more subtypes of gestational hypertension and more specific SBP and DBP ranges.
Limitations should be acknowledged when interpreting our findings. The definition of a blood pressure increment ≥ 30/15 mmHg after 20 weeks of gestation is not included in the present International Society for the Study of Hypertension in Pregnancy. The previous guidelines in China might overestimate the incidence of HDP or even be misleading regarding the risk of HDP. Since the study relied on existing data, certain unmeasured confounders, such as maternal smoking, alcohol use, antihypertensive medication, and therapeutic treatment of hypertension before or during pregnancy, could not be accounted for. However, smoking and alcohol use were both rare among women in China at the time of our study, especially among reproductive-age women living in rural areas. The results of the 1996 national smoking prevalence survey in China reported that smoking prevalence among women aged 20–29 years was <2% [25]. Other unmeasured potential confounders include certain environmental toxins, air pollution, maternal infections, and antihypertensive therapy during pregnancy. There were very low incidences of gestational hypertension and preeclampsia in some strata, thus rendering some of our analyses seriously underpowered. Our research participants were of the Han ethnicity (China’s predominant ethnic group), so our results may not be generalizable to other populations. Another limitation of the study pertained to the population selection. The original cohort included two southern provinces and one northern province. Our analysis did not include the northern province because detailed clinical records of preconception health examinations were not available. We determined from Tables in the online-only Data Supplement that the demographic characteristics of women in the final analysis (n = 45,628) differed from those of the women from the 7 included counties who were not included in this analysis (n = 40,429). It may not be appropriate to generalize our results to other populations with different demographic characteristics. As a consequence, we could not investigate the effect in the northern Chinese population. Moreover, we measured women’s blood pressure by using a cuff on their right arm with a mercury sphygmomanometer, and there could be interarm differences in blood pressure. We had no information about life changes during pregnancy and thus were unable to analyze the effect of behavioral changes on gestational hypertension and preeclampsia. Another limitation is that the women in our study were diagnosed with hypertension based on one single office visit at the registration visit, compared with 2–3 different office visits to confirm sustained elevations in blood pressure in clinical practice. This may have contributed to the lack of an association. Finally, we calculated gestational age based on menstrual dates, which could potentially lead to misclassification of gestational hypertension and preeclampsia, resulting in an underestimation of risk.
Overall, this study provides unique insight into the associations between different hypertension statuses and gestational hypertension and preeclampsia. Our findings indicate that preconception blood pressure is an important index for gestational hypertension and preeclampsia.
References
Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS ONE. 2014;9:e100180.
Skjaerven R, Wilcox AJ, Klungsoyr K, Irgens LM, Vikse BE, Vatten LJ, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677.
Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–15.
Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36:412–9.
Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22:724–30.
Magnussen EB, Vatten LJ, Myklestad K, Salvesen KA, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204:526 e1–8.
Li X, Tan H, Huang X, Zhou S, Hu S, Wang X, et al. Similarities and differences between the risk factors for gestational hypertension and preeclampsia: a population based cohort study in south China. Pregnancy Hypertens. 2016;6:66–71.
Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–90.
Gindler J, Li Z, Berry RJ, Zheng J, Correa A, Sun X, et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet. 2001;358:796–800.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52.
Whitworth JA, Chalmers J. World health organisation-international society of hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens. 2004;26:747–52.
Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. 2013;61:873–9.
Elvan-Taspinar A, Franx A, Bots ML, Koomans HA, Bruinse HW. Arterial stiffness and fetal growth in normotensive pregnancy. Am J Hypertens. 2005;18:337–41.
Tomimatsu T, Fujime M, Kanayama T, Mimura K, Koyama S, Kanagawa T, et al. Maternal arterial stiffness in normotensive pregnant women who subsequently deliver babies that are small for gestational age. Eur J Obstet Gynecol Reprod Biol. 2013;169:24–27.
Khan F, Mires G, Macleod M, Belch JJ. Relationship between maternal arterial wave reflection, microvascular function and fetal growth in normal pregnancy. Microcirculation. 2010;17:608–14.
Li N, Li Z, Ye R, Zhang L, Li H, Zhu Y, et al. Preconception blood pressure and risk of low birth weight and small for gestational age: a large cohort study in China. Hypertension. 2016;68:873–9.
Yang Y, He Y, Li Q, Wang Y, Peng Z, Xu J, et al. Preconception blood pressure and risk of preterm birth: a large historical cohort study in a Chinese rural population. Fertil Steril. 2015;104:124–30.
Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–87.
Egeland GM, Klungsoyr K, Oyen N, Tell GS, Naess O, Skjaerven R. Preconception cardiovascular risk factor differences between gestational hypertension and preeclampsia: Cohort Norway Study. Hypertension. 2016;67:1173–80.
Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978.
Everett TR, Lees CC. Beyond the placental bed: placental and systemic determinants of the uterine artery Doppler waveform. Placenta. 2012;33:893–901.
Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–9.
Thorp J.M. Placental vascular compromise: unifying the etiologic pathways of perinatal compromise. Curr Probl Obstet Gynecol Fertil. 2001;24:0202–20.
Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72:442–50.
Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282:1247–53.
Acknowledgements
The authors thank all of the volunteers and staff involved in this research.
Funding
NL was supported by the Beijing Natural Science Foundation (7194285), the National Natural Science Foundation of China (81903327), startup funding from the “Incubation” Program of China and Peking University Health Science Center (No. BMU2017YB003), and the Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001). The original project was supported by a cooperative agreement between the US Centers for Disease Control and Prevention and Peking University (Grant No. U01 DD000293).
Author information
Authors and Affiliations
Contributions
NL had full access to all of the study’s data and takes complete responsibility for the accuracy of the data analysis. Study concept and design: NL, HA, and RY. Acquisition of data: NL, HA, HL, and JL. Interpretation of data: NL, HA, ZL, LZ, and JL. Drafting of the paper: NL and HA. Critical revision of the paper: NL, HL, RY, ZL, and LZ. Statistical analysis: NL and HA. Funding acquisition: NL, JL, and RY. Administrative and technical support: NL, HA, ZL, and RY. Study supervision: ZL and RY.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, N., An, H., Li, Z. et al. Preconception blood pressure and risk of gestational hypertension and preeclampsia: a large cohort study in China. Hypertens Res 43, 956–962 (2020). https://doi.org/10.1038/s41440-020-0438-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0438-9
Keywords
This article is cited by
-
Preeclampsia up to date—What’s going on?
Hypertension Research (2023)
-
Annual reports on hypertension research 2020
Hypertension Research (2022)