Abstract
Resistant hypertension is associated with a poor prognosis due to organ damage caused by prolonged suboptimal blood pressure control. The concomitant use of mineralocorticoid receptor (MR) antagonists with other antihypertensives has been shown to improve blood pressure control in some patients with resistant hypertension, and such patients are considered to have MR-associated hypertension. MR-associated hypertension is classified into two subtypes: one with a high plasma aldosterone level, which includes primary aldosteronism (PA), and the other with a normal aldosterone level. In patients with unilateral PA, adrenalectomy may be the first-choice procedure, while in patients with bilateral PA, MR antagonists are selected. In addition, in patients with other types of MR-associated hypertension with high aldosterone levels, MR antagonists may be selected as a first-line therapy. In patients with normal aldosterone levels, ARBs or ACE inhibitors are used as a first-line therapy, and MR antagonists may be used as an add-on agent. Since MR antagonist therapy may have efficacy as a first-line or add-on agent in these patients, it is important to recognize this type of hypertension. Further studies are needed to elucidate the pathogenesis and management of MR-associated hypertension in more detail to improve the clinical outcomes of patients with MR-associated hypertension.
Similar content being viewed by others
Aldosterone and the mineralocorticoid receptor
Aldosterone is a steroid hormone and downstream effector of angiotensin II in the renin–angiotensin–aldosterone system (RAAS). Aldosterone is mainly synthesized in the adrenal cortex but is also expressed in the brain, heart, vasculature, and adipose tissues, leading to local autocrine and/or paracrine effects. The mineralocorticoid receptor (MR) is found in both epithelial and nonepithelial tissues, such as the kidney, colon, brain, heart, vasculature, and adipose tissue, and binds mineralocorticoids and glucocorticoids with equal affinity. In addition to the well-known effects of aldosterone in the kidney, including sodium reabsorption with concomitant potassium and hydrogen ion excretion that leads to blood pressure elevation, more widespread effects of aldosterone include sympathetic nervous system activation and increased oxidative stress, with inflammation, remodeling, apoptosis, and fibrosis of the cardiovascular tissues. The biological activity of MR is mediated by the differential expression of proteins resulting from the interactions of multiple complicated transcriptional and translational mechanisms [1]. In addition to the genomic effects of aldosterone, aldosterone also has nongenomic effects, whereby it acts rapidly on target tissues within several minutes [2].
Primary aldosteronism
Primary aldosteronism (PA) is an endocrine form of hypertension that was first described by Jerome Conn in 1955 in a young woman with an adrenocortical adenoma [3]. The characteristic features of PA are hypertension, increased aldosterone, and suppressed renin. Although PA was considered to be a rare disease associated with hypokalemia, which was a requisite for pursuing diagnostic work-up, it is now widely accepted that PA is the most common form of endocrine hypertension, with the majority of patients displaying normokalaemia [4].
The estimated prevalence of PA is 4–6% among patients with hypertension in primary care, ~10% in specialized hypertensive clinics, and 20% in patients with resistant hypertension [5], which is defined as persistent hypertension with concomitant use of three different antihypertensive drugs, including a diuretic [6].
Patients with PA have an increased risk of cardiovascular and cerebrovascular events, heart [7, 8] and renal diseases [8, 9], diabetes mellitus, and metabolic syndrome [8, 10] and a reduced quality of life [11, 12]. These observations indicate the importance of an early diagnosis and appropriate treatment of PA with specific surgical or medical treatment, as described below.
Aldosterone and the MR in cardiovascular complications in non-PA patients
Clinical studies have shown a relationship between plasma aldosterone levels and left ventricular hypertrophy (LVH), renal injury, vascular disease [13,14,15,16,17,18], atrial flutter and atrial fibrillation [19], and structural and functional alterations of medium-caliber arteries [20, 21], as well as microcirculation injuries and alterations in endothelial function [22,23,24,25,26,27,28].
In addition, the beneficial effects of pharmacological MR blockade have been clearly demonstrated in cardiovascular disease (CVD) and chronic kidney disease (CKD). The RALES [29], EPHESUS [30], and EMPHASIS-HF [31] studies have shown the benefits of MR antagonists in patients with moderate-to-severe congestive heart failure, suggesting that excessive activation of MR is involved in the pathophysiology of heart failure. Serum aldosterone levels were elevated in patients with chronic atrial fibrillation and decreased rapidly after electrical cardioversion [32]. MR antagonists showed beneficial effects in dialysis patients [33] or those who underwent cardiac surgery [34], with a reduction in the occurrence of atrial fibrillation. MR antagonists reduced the risk of rhythm disorders and cardiac arrest in patients with heart failure [35] or myocardial infarction [36]. In addition, meta-analyses showed a substantial antiproteinuric effect and a possible major renoprotective effect of MR antagonists [37, 38].
MR-associated hypertension
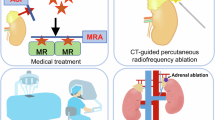
Resistant hypertension is associated with a poor prognosis because of organ damage caused by prolonged suboptimal blood pressure control and has associations with diabetes mellitus, CKD, and obesity [39, 40]. Resistant hypertension is increasingly common in clinical practice, and treatment focuses on maximizing the doses of antihypertensive drugs and adding drugs with complementary mechanisms of action, including a combination of calcium channel blockers, angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors, and diuretics [6] (Table 1). The concomitant use of an MR antagonist with other antihypertensives has been shown to improve blood pressure control in some patients with resistant hypertension (Table 2) [41,42,43], known as MR-associated hypertension [44] (Fig. 1).
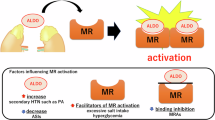
MR-associated hypertension is classified into two subtypes, one with a high plasma aldosterone level and the other with a normal aldosterone level [44]. In the former subtype, the plasma aldosterone level is relatively high (usually ≥ 150 pg/ml) in proportion to the plasma renin activity. It is logical that MR antagonists are effective in the treatment of resistant hypertension with elevated plasma aldosterone levels; however, it is notable that several subsets of patients with resistant hypertension and normal plasma aldosterone levels can also be effectively controlled by add-on therapy with MR antagonists [44].
MR-associated hypertension with elevated plasma aldosterone levels
PA is a typical type of MR-associated hypertension with elevated plasma aldosterone levels. In addition to PA, an elevated plasma aldosterone concentration has been implicated in several other forms of hypertension.
Aldosterone breakthrough (escape) phenomenon
Renin–angiotensin system (RAS) inhibitors reduce plasma aldosterone levels in the initial treatment phase, but aldosterone levels may later reach, or sometimes exceed, pretreatment values [45, 46]. This phenomenon is known as “aldosterone breakthrough” or “aldosterone escape” and may limit the beneficial effects of RAS inhibitors. It has been shown that aldosterone breakthrough is independent of RAS inhibitor dosage [47] and does not differ with ACE inhibitor or ARB treatment [48]. Aldosterone breakthrough may be associated with cardiovascular and renal morbidity. Indeed, it has been reported to reverse the beneficial effects of an ACE inhibitor on LVH [49], and the improvement in functional capacity noted in patients with congestive heart failure treated with an ACE inhibitor was decreased when aldosterone breakthrough was present [50]. In patients with CVD and CKD exhibiting aldosterone breakthrough, the addition of an MR antagonist significantly restored the organ-protective effects of RAS inhibitors without changing blood pressure [51, 52]. These data suggested that inappropriately elevated plasma aldosterone levels play a critical role in the pathogenesis of CVD and CKD in high-risk patients who are treated with a RAS inhibitor in the long term.
Aldosterone-associated hypertension
Clinical and biochemical evidence has indicated the existence of a gray zone between patients with essential hypertension and PA, leading to a hypothesis that there is a continuum, which includes essential hypertension, low-renin hypertension, and bilateral idiopathic hyperaldosteronism (IHA) [53, 54]. Patients with an elevated aldosterone-to-renin ratio (ARR) and plasma aldosterone level are more likely to have resistant hypertension than are patients with essential hypertension, even when they do not meet the diagnostic criteria for PA [55]. Such hypertension is defined as “aldosterone-associated hypertension” and is likely to be a resistant type of hypertension.
Obesity
Aldosterone excess has been implicated in obesity-related disorders [56, 57]. Indeed, obese women have been reported to have higher aldosterone levels than age-matched lean female controls, with aldosterone levels in these two groups becoming comparable after weight loss in the obese group [57, 58]. A correlation between plasma aldosterone levels and fat mass was observed in normotensive women, raising the possibility that there is an effect of adipose tissue on aldosterone production [57]. Furthermore, aldosterone production was shown to be exclusively correlated with subcutaneous white adipose tissue [59]. In addition, leptin released from adipocytes is directly associated with the release of aldosterone from adipose tissue [60]. Interestingly, secretory products from isolated human adipocytes strongly stimulated aldosterone secretion in human adrenocortical cells, indicating that human adipocytes secrete aldosterone-releasing factors [56, 61]. These data may suggest that increased aldosterone secretion from adipose tissue and/or the adrenal gland via adipocyte-derived aldosterone-releasing factors may play an important role in MR-associated hypertension with elevated plasma aldosterone levels in the context of obesity.
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is the secondary condition most frequently associated with resistant hypertension [62]. Taking into account moderate and severe forms of OSA, the prevalence of OSA among patients with resistant hypertension ranges from 45% to 80% and has been reported to be up to 95% [62,63,64,65,66]. There is growing evidence to support the role of aldosterone in OSA; specifically, high levels of serum aldosterone appear to predispose resistant hypertensive patients to OSA through a proposed mechanism of peripharyngeal fluid retention [67]. Medical management using spironolactone, an MR antagonist, improved OSA symptoms in patients with resistant hypertension [68]. In addition, both surgical and medical treatments for PA were associated with improvements in OSA severity [69].
Sleep disorders
A growing number of epidemiologic studies have indicated that shift workers, long-distance transmeridian flight crews, and patients with sleep disorders show a higher than average prevalence of hypertension-derived CVD [70,71,72]. Moreover, a clinical trial demonstrated an increase in blood pressure in healthy volunteers following placement in an environment that induced circadian rhythm abnormalities [73]. Melatonin is involved in the physiological control of circadian rhythms, as well as in the activities of hormones and cytokines [74,75,76]. Most sleep disorders are associated with a disrupted circadian rhythm, in which the reduction of melatonin secretion leads to biological clock dysfunction. Mice lacking the core clock genes Cry1 and Cry2 displayed abnormalities of circadian rhythm and salt-sensitive hypertension due to chronic overproduction of aldosterone by the adrenal gland [77]. These data suggest that sleep disorders are associated with the pathogenesis of MR-associated hypertension.
MR-associated hypertension with normal plasma aldosterone levels
Diabetes mellitus
Hypertension is present in more than 50% of patients with diabetes mellitus and contributes significantly to both microvascular and macrovascular diseases in diabetes mellitus. ACE inhibitors or ARBs are recommended as first-line antihypertensive therapies for diabetes mellitus, and both drugs have been shown to have renoprotective effects [78, 79]. However, blood pressure control using ACE inhibitors or ARBs is often difficult in patients with diabetes mellitus, and treatment with multiple drugs with different mechanisms is necessary for blood pressure reduction [80]. Indeed, spironolactone has been shown to have beneficial effects in patients previously treated with ACE inhibitors or ARBs [81, 82]. Furthermore, the addition of spironolactone to a regimen that includes a maximal dose of an ACE inhibitor showed greater renoprotection than the addition of an ARB in the context of diabetic nephropathy, indicating that MR is overactivated in selected diabetic patients, even in the absence of high plasma aldosterone levels [79, 83].
Chronic kidney disease
There is considerable evidence to suggest that RAAS plays an important role in the pathogenesis and progression of renal diseases. Blockade of RAAS by ACE inhibitors or ARBs is considered the most effective therapy for slowing the progression of CKD. Therefore, ACE inhibitors or ARBs have become first-line therapies for the management of patients with proteinuric CKD in a manner similar to diabetes mellitus. It is speculated that a decrease in sodium excretion may cause extracellular volume expansion to decrease the levels of renin and aldosterone in CKD. However, elevated aldosterone levels have been demonstrated in both animals with kidney dysfunction [84] and patients with CKD [85], which could be explained by multiple factors that promote hyperaldosteronism in CKD [86]. Many CKD patients have obesity and/or OSA, conditions associated with elevated aldosterone levels. CKD patients often have hyperkalemia, which directly stimulates aldosterone secretion. Furthermore, elevated renin secretion due to renal ischemia or glomerulonephritis may increase aldosterone levels via increased angiotensin II production. Therefore, the addition of an MR antagonist to an ACE inhibitor or ARB is recommended because of the possible activation of MR, in addition to aldosterone breakthrough caused by ACE inhibitors or ARBs in CKD [83, 87, 88]; indeed, beneficial effects of MR antagonists in CKD have been reported [38, 86, 89]. Accordingly, it is suggested that direct activation of the MR in pathological states is associated with CKD, even in the absence of a prominent increase in plasma aldosterone levels [44].
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a common disease affecting ~10% of women of reproductive age. PCOS is characterized by oligoanovulation and clinical/biochemical signs of hyperandrogenism, and although several phenotypes have been described, they share a common feature of insulin resistance [90]. Beyond reproductive disorders, even at an early stage, PCOS patients have a clustering of cardiovascular risk factors, such as hypertension, insulin resistance, obesity, diabetes mellitus, dyslipidemia, endothelial dysfunction, and low-grade chronic inflammation [91]. Serum aldosterone levels and ARR, though normal and not consistent with PA, were reported to be higher in PCOS women than in age- and body mass index-matched healthy controls and to correlate with blood pressure and some metabolic and cardiovascular markers [92, 93]. Insulin resistance is considered a cause of the increased aldosterone levels, even though they remain within normal limits, in PCOS [44].
MR overstimulation by other factors
Glucocorticoids
The affinity of MR for aldosterone and glucocorticoids is similar, but the plasma glucocorticoid level is 1000 times greater than that of aldosterone [94]. Although 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) transforms glucocorticoids into inactive metabolites, several studies have indicated that physiological levels of glucocorticoids activate MR under pathophysiological conditions [95]. Furthermore, high serum cortisol levels are observed in Cushing’s syndrome, and glucocorticoids are increased in subjects with obesity, diabetes mellitus, and inflammation [96]. These data suggest that inappropriate activation of MR might be induced by glucocorticoids during the development of lifestyle-related diseases [97]. Consistent with this hypothesis, glucocorticoid-induced MR activation mediates renal injury in high-salt-treated adrenalectomized rats [94].
RAC-1
It has been shown that excessive salt intake induces renal injury, which is ameliorated by blockade of MR, suggesting that renal MR is pathologically activated even when the plasma aldosterone level is suppressed. A recent study showed that the small guanosine triphosphatase (GTPase) Rac1 increases the nuclear translocation of MR, resulting in enhanced MR activity [98, 99]. Rac1 is activated by several factors, including cytokines [100], mechanical stress [101], dietary high-salt intake [102], and oxidative stress [103], all of which are risk factors for CVD and CKD. Therefore, it is considered that during the development of CVD and CKD, MR is activated by Rac1-dependent pathways, which further increases the risk of CVD and CKD.
Alteration of MR status
MR activation by aldosterone-independent mechanisms has been implicated in some pathophysiological conditions. These mechanisms could involve alteration of MR activity, which may cause MR-associated hypertension. These mechanisms were reported to include increases in MR gene transcription, MR sensitivity, MR stabilization, and/or MR overstimulation via activating mutations of the MR gene [44].
Management of MR-associated hypertension
Surgical adrenalectomy
In patients with unilateral PA, adrenalectomy is the first-choice procedure. This procedure is now primarily performed laparoscopically, which has resulted in a lower complication rate and shorter hospitalizations [104,105,106]. After surgery, a biochemical cure may be achieved; however, the postoperative blood pressure normalization rate is ~30% due to the duration and severity of hypertension and the presence of essential hypertension.
Data from direct comparisons of surgical adrenalectomy and medical treatment with MR antagonists in the treatment of PA are limited due to bias related to the varied demographics and clinical presentation of unilateral versus bilateral disease. However, a small number of studies have shown better long-term cardiovascular outcomes, renal outcomes, and mortality with surgical adrenalectomy than with MR antagonist therapy [107,108,109]. In patients with unilateral PA who are willing and able to safely undergo surgery, adrenalectomy may be recommended as the preferred treatment approach.
Dietary sodium restriction
Because MR-associated hypertension is a form of salt-sensitive resistant hypertension, dietary sodium restriction should be encouraged in patients with both PA and MR-associated hypertension. Restriction of dietary sodium intake can result in volume contraction, leading to an increase in both renin and angiotensin II. Increased angiotensin II leads to decreased distal sodium delivery, thereby limiting the pathologic consequences of aldosterone-MR-epithelial sodium channel (ENaC)-mediated distal sodium reabsorption.
MR antagonists
Lifelong MR antagonist therapy is recommended for patients with bilateral PA, as well as those with unilateral PA who are unable to or unwilling to undergo surgical adrenalectomy. MR antagonists decrease ENaC-mediated sodium reabsorption and consequent volume expansion and reduce potassium and hydrogen ion excretion, subsequently leading to a substantial reduction in blood pressure (or a decrease in antihypertensive medication) and an improvement in potassium balance.
As a therapeutic approach for resistant hypertension with high plasma aldosterone levels, MR antagonists are recommended as first-line drugs [44]. In medical treatment for resistant hypertension, a combination of a calcium channel blocker, an ARB or ACE inhibitor and a diuretic is generally used [6]. Furthermore, when the target blood pressure cannot be achieved using these three drugs, additional administration of an MR antagonist is recommended (Table 1) [6]. In particular, ARBs or ACE inhibitors are frequently used as first-line drugs to control hypertension in patients with MR-associated hypertension, including obesity, diabetes mellitus, CKD, and PCOS [87, 88]. Based on the pathogenesis of MR-associated hypertension, MR antagonists should be given as an add-on agent for the treatment of resistant hypertension with normal plasma aldosterone levels.
Regarding individual MR antagonists, spironolactone is a nonselective, competitive MR antagonist that is structurally similar to progesterone and metabolized to active metabolites in the liver. Additionally, spironolactone also acts as an antagonist to the androgen receptor, a weak antagonist to the glucocorticoid receptor and an agonist to the progesterone receptor. These receptor-mediated actions also result in adverse effects of spironolactone, including hyperkalemia, hyponatremia, gynecomastia, impotence, menstrual disturbances, hirsutism, and decreased libido, which are regarded as clinically relevant problems.
Eplerenone is derived from spironolactone and is considered a selective MR antagonist that has limited cross-reactivity for the androgen and progesterone receptors and thus lacks many of the significant sexually related adverse effects known to be associated with the use of spironolactone. However, its affinity for MR is low, and it exhibits weak MR antagonism. In Japan and the United States, eplerenone is contraindicated in hypertensive patients with diabetes mellitus and concomitant albuminuria, microalbuminuria, or proteinuria or in patients with a creatinine clearance of <50 mL/min due to the considerable risk of increased serum K+ levels seen in clinical studies [110, 111].
Esaxerenone is a novel oral, nonsteroidal, and selective MR antagonist that undergoes hepatic metabolism and has a long half-life. The MR affinity of esaxerenone is 4- and 76-fold greater than that of spironolactone and eplerenone, respectively, and the half-maximal inhibitory concentration (IC50) of esaxerenone for the transcriptional activity of human MR is 3.7, compared with 66 and 970 nmol/L for spironolactone and eplerenone, respectively [112]. Recently, the antihypertensive activity of esaxerenone 5 mg/day was shown to be superior to that of eplerenone 50 mg/day in a double-blind randomized Phase 3 study [113].
Conclusion
Hypertension in which increased MR activity is associated with blood pressure elevation and MR antagonists are effective in reducing blood pressure is considered to be MR-associated hypertension [44]. MR-associated hypertension is classified into two subtypes, one with high plasma aldosterone levels, which includes PA, and the other with normal aldosterone levels. In patients with unilateral PA, adrenalectomy may be the first-choice procedure, while in patients with bilateral PA, as well as those with unilateral PA who are unable to or unwilling to undergo adrenalectomy, MR antagonist therapy may be recommended. In addition, in patients with other types of MR-associated hypertension with high aldosterone levels, MR antagonists may be selected as a first-line therapy; in those with normal aldosterone levels, ARBs or ACE inhibitors are used as a first-line therapy, and MR antagonists may be recommended as an add-on agent. Because MR antagonist therapy may be effective as a first-line or add-on agent in these patients, an attempt to recognize these conditions may be required. Furthermore, future studies are required to investigate the pathogenesis and management of MR-associated hypertension in more detail to improve the clinical outcomes of patients with MR-associated hypertension.
References
Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012.
Funder JW. Minireview: aldosterone and the cardiovascular system: genomic and nongenomic effects. Endocrinology. 2006;147:5564–7.
Conn JW. Primary aldosteronism. J Lab Clin Med. 1955;45:661–4.
Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–50.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–20.
Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab. 2009;94:1191–7.
Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High prevalence of diabetes in patients with primary aldosteronism (pa) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a large, multicenter cohort study in Japan. Diabetes Care. 2019;42:938–45.
Velema M, Dekkers T, Hermus A, Timmers H, Lenders J, Groenewoud H, et al. Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J Clin Endocrinol Metab. 2018;103:16–24.
Reincke M. Anxiety, depression, and impaired quality of life in primary aldosteronism: why we shouldn’t ignore it! J Clin Endocrinol Metab. 2018;103:1–4.
Rocha R, Stier CT Jr., Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–8.
El-Gharbawy AH, Nadig VS, Kotchen JM, Grim CE, Sagar KB, Kaldunski M, et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–50.
Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci. 2002;970:89–100.
Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–800.
Rossi GP, Cesari M, Pessina AC. Left ventricular changes in primary aldosteronism. Am J Hypertens. 2003;16:96–8.
Dartsch T, Fischer R, Gapelyuk A, Weiergraeber M, Ladage D, Schneider T, et al. Aldosterone induces electrical remodeling independent of hypertension. Int J Cardiol. 2013;164:170–8.
Rossi GP, Seccia TM, Gallina V, Muiesan ML, Leoni L, Pengo M, et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens. 2013;27:158–63.
Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, et al. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension. 2008;51:1366–71.
Martinez-Aguayo A, Carvajal CA, Campino C, Aglony M, Bolte L, Garcia H, et al. Primary aldosteronism and its impact on the generation of arterial hypertension, endothelial injury and oxidative stress. J Pediatr Endocrinol Metab. 2010;23:323–30.
Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–7.
Rocha R, Stier CT Jr. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308–14.
Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–10.
Takeda Y. Role of cardiovascular aldosterone in hypertension. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:261–6.
Yugar-Toledo JC, Bonalume Tacito LH, Ferreira-Melo SE, Sousa W, Consolin-Colombo F, Irigoyen MC, et al. Low-renin (volume dependent) mild-hypertensive patients have impaired flow-mediated and glyceryl-trinitrate stimulated vascular reactivity. Circ J. 2005;69:1380–5.
Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–8.
Ruilope LM. Aldosterone, hypertension, and cardiovascular disease: an endless story. Hypertension. 2008;52:207–8.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
Goette A, Hoffmanns P, Enayati W, Meltendorf U, Geller JC, Klein HU. Effect of successful electrical cardioversion on serum aldosterone in patients with persistent atrial fibrillation. Am J Cardiol. 2001;88:906–9, A8.
Chung YW, Yang YH, Wu CK, Yu CC, Juang JM, Wang YC, et al. Spironolactone is associated with reduced risk of new-onset atrial fibrillation in patients receiving renal replacement therapy. Int J Cardiol. 2016;202:962–6.
Simopoulos V, Tagarakis G, Hatziefthimiou A, Skoularigis I, Triposkiadis F, Trantou V, et al. Effectiveness of aldosterone antagonists for preventing atrial fibrillation after cardiac surgery in patients with systolic heart failure: a retrospective study. Clin Res Cardiol. 2015;104:31–7.
Wei J, Ni J, Huang D, Chen M, Yan S, Peng Y. The effect of aldosterone antagonists for ventricular arrhythmia: a meta-analysis. Clin Cardiol. 2010;33:572–7.
Beygui F, Labbe JP, Cayla G, Ennezat PV, Motreff P, Roubille F, et al. Early mineralocorticoid receptor blockade in primary percutaneous coronary intervention for ST-elevation myocardial infarction is associated with a reduction of life-threatening ventricular arrhythmia. Int J Cardiol. 2013;167:73–9.
Bolignano D, Palmer SC, Navaneethan SD, and Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004.
Currie G, Taylor AH, Fujita T, Ohtsu H, Lindhardt M, Rossing P, et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17:127.
Myat A, Redwood SR, Qureshi AC, Spertus JA, Williams B. Resistant hypertension. BMJ. 2012;345:e7473.
Williams B. Resistant hypertension: an unmet treatment need. Lancet. 2009;374:1396–8.
Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376–85.
Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25:514–23.
Bertocchio JP, Warnock DG, Jaisser F. Mineralocorticoid receptor activation and blockade: an emerging paradigm in chronic kidney disease. Kidney Int. 2011;79:1051–60.
Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens. 2003;16:781–8.
MacFadyen RJ, Lee AF, Morton JJ, Pringle SD, Struthers AD. How often are angiotensin II and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart. 1999;82:57–61.
Horita Y, Taura K, Taguchi T, Furusu A, Kohno S. Aldosterone breakthrough during therapy with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in proteinuric patients with immunoglobulin A nephropathy. Nephrology. 2006;11:462–6.
Sato A, Saruta T. Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in essential hypertensive patients with left ventricular hypertrophy. J Int Med Res. 2001;29:13–21.
Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zeni P, et al. Relation of aldosterone “escape” despite angiotensin-converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;89:403–7.
Narayan H, Webb DJ. New evidence supporting the use of mineralocorticoid receptor blockers in drug-resistant hypertension. Curr Hypertens Rep. 2016;18:34.
Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res. 2006;29:211–6.
Kaplan NM. The current epidemic of primary aldosteronism: causes and consequences. J Hypertens. 2004;22:863–9.
Racine MC, Douville P, Lebel M. Functional tests for primary aldosteronism: value of captopril suppression. Curr Hypertens Rep. 2002;4:245–9.
Sartori M, Calo LA, Mascagna V, Realdi A, Macchini L, Ciccariello L, et al. Aldosterone and refractory hypertension: a prospective cohort study. Am J Hypertens. 2006;19:373–9.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA. 2003;100:14211–6.
Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fat Acids. 1999;60:401–5.
Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–62.
Harada E, Mizuno Y, Katoh D, Kashiwagi Y, Morita S, Nakayama Y, et al. Increased urinary aldosterone excretion is associated with subcutaneous not visceral, adipose tissue area in obese individuals: a possible manifestation of dysfunctional subcutaneous adipose tissue. Clin Endocrinol. 2013;79:510–6.
Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;132:2134–45.
Krug AW, Vleugels K, Schinner S, Lamounier-Zepter V, Ziegler CG, Bornstein SR, et al. Human adipocytes induce an ERK1/2 MAP kinases-mediated upregulation of steroidogenic acute regulatory protein (StAR) and an angiotensin II-sensitization in human adrenocortical cells. Int J Obes. 2007;31:1605–16.
Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–7.
Florczak E, Prejbisz A, Szwench-Pietrasz E, Sliwinski P, Bielen P, Klisiewicz A, et al. Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens. 2013;27:678–85.
Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7.
Martinez-Garcia MA, Navarro-Soriano C, Torres G, Barbe F, Caballero-Eraso C, Lloberes P, et al. Beyond resistant hypertension. Hypertension. 2018;72:618–24.
Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8.
Sim JJ, Yan EH, Liu IL, Rasgon SA, Kalantar-Zadeh K, Calhoun DA, et al. Positive relationship of sleep apnea to hyperaldosteronism in an ethnically diverse population. J Hypertens. 2011;29:1553–9.
Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–7.
Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31:561–7.
Furlan R, Barbic F, Piazza S, Tinelli M, Seghizzi P, Malliani A. Modifications of cardiac autonomic profile associated with a shift schedule of work. Circulation. 2000;102:1912–6.
Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–6.
Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, et al. Shift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort study. Hypertension. 2008;52:581–6.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–8.
Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127–51.
Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10.
Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–9.
Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74.
Grossman E, Messerli FH. Management of blood pressure in patients with diabetes. Am J Hypertens. 2011;24:863–75.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–50.
Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–14.
van den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D, et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24:2285–92.
Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–8.
Jansen PM, Danser AH, Imholz BP, van den Meiracker AH. Aldosterone-receptor antagonism in hypertension. J Hypertens. 2009;27:680–91.
Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Investig. 1996;98:1063–8.
Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–23.
Volk MJ, Bomback AS, Klemmer PJ. Mineralocorticoid receptor blockade in chronic kidney disease. Curr Hypertens Rep. 2011;13:282–8.
Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211.
Bomback AS, Toto R. Dual blockade of the renin-angiotensin-aldosterone system: beyond the ACE inhibitor and angiotensin-II receptor blocker combination. Am J Hypertens. 2009;22:1032–40.
Toto RD. Aldosterone blockade in chronic kidney disease: can it improve outcome? Curr Opin Nephrol Hypertens. 2010;19:444–9.
Zulian E, Sartorato P, Benedini S, Baro G, Armanini D, Mantero F, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Investig. 2005;28:49–53.
Dona G, Sabbadin C, Fiore C, Bragadin M, Giorgino FL, Ragazzi E, et al. Inositol administration reduces oxidative stress in erythrocytes of patients with polycystic ovary syndrome. Eur J Endocrinol. 2012;166:703–10.
Cascella T, Palomba S, Tauchmanova L, Manguso F, Di Biase S, Labella D, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91:4395–400.
Armanini D, Bordin L, Dona G, Sabbadin C, Bakdounes L, Ragazzi E, et al. Polycystic ovary syndrome: implications of measurement of plasma aldosterone, renin activity and progesterone. Steroids. 2012;77:655–8.
Rafiq K, Hitomi H, Nakano D, Nishiyama A. Pathophysiological roles of aldosterone and mineralocorticoid receptor in the kidney. J Pharm Sci. 2011;115:1–7.
Baker ME, Funder JW, Kattoula SR. Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol. 2013;137:57–70.
Vitellius G, Trabado S, Bouligand J, Delemer B, Lombes M. Pathophysiology of glucocorticoid signaling. Ann Endocrinol. 2018;79:98–106.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Uddin S, Lekmine F, Sharma N, Majchrzak B, Mayer I, Young PR, et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J Biol Chem. 2000;275:27634–40.
Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Zhu W, et al. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ Res. 1999;84:458–66.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Investig. 2011;121:3233–43.
Nagase M, Ayuzawa N, Kawarazaki W, Ishizawa K, Ueda K, Yoshida S, et al. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: role of small GTPase Rac1. Hypertension. 2012;59:500–6.
Duncan JL 3rd, Fuhrman GM, Bolton JS, Bowen JD, Richardson WS. Laparoscopic adrenalectomy is superior to an open approach to treat primary hyperaldosteronism. Am Surg. 2000;66:932–5. discussion 935–6
Meria P, Kempf BF, Hermieu JF, Plouin PF, Duclos JM. Laparoscopic management of primary hyperaldosteronism: clinical experience with 212 cases. J Urol. 2003;169:32–5.
Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg. 2002;68:253–6. discussion 256–7
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–66.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–74.
Pelliccia F, Patti G, Rosano G, Greco C, Gaudio C. Efficacy and safety of eplerenone in the management of mild to moderate arterial hypertension: systematic review and meta-analysis. Int J Cardiol. 2014;177:219–28.
Roush GC, Ernst ME, Kostis JB, Yeasmin S, Sica DA. Dose doubling, relative potency, and dose equivalence of potassium-sparing diuretics affecting blood pressure and serum potassium: systematic review and meta-analyses. J Hypertens. 2016;34:11–9.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-Blind Randomized Phase 3 Study Comparing Esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertension. 2020;75:51–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morimoto, S., Ichihara, A. Management of primary aldosteronism and mineralocorticoid receptor-associated hypertension. Hypertens Res 43, 744–753 (2020). https://doi.org/10.1038/s41440-020-0468-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0468-3
Keywords
This article is cited by
-
Appropriate mineralocorticoid receptor antagonism and salt restriction are essential for primary aldosteronism therapy
Hypertension Research (2023)
-
Aldosterone breakthrough from a pharmacological perspective
Hypertension Research (2022)
-
Lysine-specific demethylase 1 as a corepressor of mineralocorticoid receptor
Hypertension Research (2022)
-
Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism
Hypertension Research (2021)
-
Hypothalamus-pituitary-adrenal Axis in Glucolipid metabolic disorders
Reviews in Endocrine and Metabolic Disorders (2020)