Abstract
Hypertension associated with hyperhomocysteinemia (HHcy) is associated with a high risk of vascular diseases. However, the mechanisms of HHcy-associated hypertensive renal damage and the efficacy of folic acid (FA) as a treatment have not been fully elucidated. The aim of the present study was to evaluate whether lowering the plasma homocysteine (Hcy) level using different doses of FA can reduce HHcy-associated glomerular injury in spontaneously hypertensive rats (SHRs) and to clarify the potential mechanisms of such effects. SHRs were randomized into a control group, HHcy group, HHcy + low-dose FA (LFA) group, and HHcy + high-dose FA (HFA) group. Compared with the control group, the HHcy group had reduced serum superoxide dismutase and GFR levels and elevated serum malondialdehyde and urinary albumin creatinine ratio levels. Increased extracellular matrix of the glomerulus and an increased glomerular sclerosis index, podocyte foot process effacement and fusion, as well as increased podocyte apoptosis, were observed in the HHcy group compared with the control group; these effects were associated with increased expression of NOX2 and NOX4 and decreased nephrin expression in renal tissue from SHRs with HHcy. HHcy-induced changes were counteracted by LFA and HFA treatment. Apart from lower levels of NOX2 in the HHcy + HFA group, there were no significant differences in other indicators between the HHcy + LFA and HHcy + HFA groups. These results suggest that even at a low dose, FA can reduce plasma Hcy and attenuate HHcy-induced glomerular injury by inhibiting oxidative stress and apoptosis.
Similar content being viewed by others
Introduction
Hyperhomocysteinemia (HHcy) is defined as a homocysteine (Hcy) level above 10 μmol/l in human blood [1]. Hypertension associated with HHcy has a high incidence in China, accounting for approximately three-quarters of patients with hypertension [2]. A study of 19 centers in nine European countries demonstrated that high Hcy levels and hypertension are synergistic factors that increase the risk of vascular disease [3]. Several recent studies have also found that the risk of stroke and death significantly increase when hypertension co-occurs with HHcy [4, 5]. However, little is known about the pathogenic role of HHcy in the progression of glomerular damage associated with hypertension. In a previous clinical study, we confirmed that plasma Hcy and the C677T polymorphism in the gene encoding its key metabolic enzyme, MTHFR, are independent risk factors for early renal damage in the hypertensive Chinese Han population [6]. A case–control study also indicated that HHcy may increase the susceptibility of subjects with essential hypertension to microalbuminuria [7]. However, the mechanisms of HHcy-associated hypertensive renal damage have not been fully elucidated.
Hypertension is an independent risk factor for glomerular injury. It is unclear whether HHcy increases renal damage by exacerbating hypertension or by other mechanisms independent of hypertension. Numerous studies have shown that HHcy plays a pathological role by enhancing oxidative stress in local tissues or cells. Nicotinamide adenine dinucleotide phosphate oxidase (Nox) was the first enzyme found to produce reactive oxygen species. Nox is a multicomponent enzyme that includes seven subtypes: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2 [8, 9]. NOX2 and NOX4 are widely expressed in glomerular cells (mesangial cells, podocytes), renal tubular cells, and renal interstitial cells [10, 11] and act as aerobic receptors. Recent studies have demonstrated that Nox is involved in progressive glomerular injury associated with HHcy [12, 13] and hypertension target organ damage [14]. However, it is unknown whether increased NOX2 and NOX4 expression in HHcy-associated hypertension is involved in podocyte injury and the associated pathological changes. Nephrin, a transmembrane protein located in the slit diaphragm, is an important structural molecule and signaling molecule of glomerular podocytes. It plays an important role in determining the shape and viability of podocytes and maintaining the integrity of the filtration function of the glomerulus [15, 16]. To confirm the effects of Hcy on podocyte injury in hypertension, we determined whether an increased plasma Hcy level induces the expression of Nox subunits and decreases the expression of nephrin, thereby affecting the survival of podocytes.
Currently, the safest and most effective means of reducing Hcy is folic acid (FA) supplementation, which can reduce Hcy levels in blood but also antagonize the toxic effects of Hcy [17, 18]. The China Stroke Primary Prevention Trial concluded that FA supplementation in hypertensive patients can slow chronic renal impairment [2]. Moreover, FA therapy may significantly reduce the mortality risk associated with heavy proteinuria among adults with hypertension [19]. However, the mechanism by which FA improves renal function in hypertensive patients associated with HHcy is unknown, and there is no consensus on the efficacy of FA at different doses [20,21,22]. Therefore, using an animal model of hypertension associated with HHcy, the present study investigated whether Hcy can induce downregulation of nephrin expression by enhancing oxidative stress, resulting in podocyte apoptosis and glomerular injury. The effects of high-dose (HFA) and low-dose FA (LFA) were also evaluated.
Materials and methods
Animals
Male spontaneously hypertensive rats (SHRs) (250–270 g, 12 weeks) were provided by the Beijing Vital River Laboratory Animal Center (Beijing, China). Four rats were housed per cage and given free access to food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee and carried out in full accordance with the institutional animal care and use protocols of Laboratory Animals of Shandong University.
The SHRs were randomized into a control group, HHcy group, HHcy + LFA group and HHcy + HFA group, with eight animals per group. All animals were given intraperitoneal injection for 12 weeks and gavage at the same time during the last 8 weeks of the experiment, and the SHRs were maintained as follows: (1) the control group was given physiological saline (PS, 5 ml/kg, twice a day) intraperitoneal injection for 12 weeks and PS (0.5 ml/d) by gavage for 8 weeks; (2) the HHcy group was given 2% DL-Hcy (5 ml/kg, twice a day, H4628, Sigma-Aldrich, St. Louis, USA) intraperitoneal injection for 12 weeks and PS (0.5 ml/d) by gavage for 8 weeks; (3) the HHcy + LFA group was given 2% DL-Hcy (5 ml/kg, twice a day) intraperitoneal injection for 12 weeks and FA (0.4 mg/kg/d, F7876, Sigma) by gavage for 8 weeks; and (4) the HHcy + HFA group was given 2% DL-Hcy (5 ml/kg, twice a day) intraperitoneal injection for 12 weeks and FA (4 mg/kg/d) by gavage for 8 weeks. FA was freshly dissolved in 0.5 ml of PS immediately before the experiment. Blood pressure (BP) and plasma Hcy levels were measured at weeks 4, 8, and 12. At week 12, the urine and vena cava blood of rats were collected for urine albumin, urine creatinine, plasma creatinine, serum malondialdehyde (MDA), and superoxide dismutase (SOD) detection, and renal tissues were collected for histopathological examination, immunohistochemistry, quantitative real-time polymerase chain reaction (qRT-PCR), western blotting and TUNEL detection.
Measurement of BP
The systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the tail artery were monitored in the morning with a noninvasive BP measurement system in the conscious state. To ensure the precision of the measurement, the temperature was controlled at 37–39 °C and BP was measured after the rats were quiet and the pulse was stable. The BP of each rat was measured three times and averaged.
Plasma Hcy and creatinine analysis
At weeks 4 and 8 of the experiment, rat tail blood was collected after centrifugation for the detection of plasma Hcy. At week 12 of the experiment, rats were fasted overnight and anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Blood was collected from the vena cava. The plasma Hcy and creatinine concentrations were detected using a Cobas8000 automatic biochemistry analyzer (Roche, Switzerland).
Serum oxidative stress biomarker analysis
Serum MDA and SOD levels were determined using commercial kits (Jiancheng Institute of Biological Technology, Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
Urinary albumin measurement and glomerular filtration rate (GFR)
To collect urine samples, rats were placed in metabolic cages to collect urine for 24 h and record urine volume. Urinary albumin and urine creatinine were examined by using a Cobas8000 automatic biochemistry analyzer (Roche, Switzerland). To eliminate the impact of urine volume, urinary albumin was expressed as the urinary albumin creatinine ratio (UACR). The GFR was calculated by the following equation: GFR = (urine creatinine/plasma creatinine) × urine volume/body weight [23].
qRT-PCR
Total RNA was extracted from rat kidney tissue with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse transcribed to cDNA with a Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). Primer sequences (BioSune Biotechnology, Shanghai, China) are listed in Table 1. The mRNA levels were analyzed with an ABI ViiA 7 Real-Time PCR system (Applied Biosystems, Foster City, CA) using SYBR Green Real-Time PCR reagent (Roche, Indianapolis, IN, USA). Gene expression levels were normalized to GAPDH expression levels. The relative quantity of mRNA expression was calculated according to the cycle threshold (\(2^{-\Delta\Delta {\mathrm{Ct}}}\)) method.
Western blot analysis
The kidney cortical tissues were cut into pieces, and total protein was extracted using RIPA protein extraction solution. Homogenates were incubated on ice for 30 min and centrifuged at 12,000 × g for 10 min at 4 °C. Protein concentrations were measured using an enhanced BCA protein assay kit (Beyotime, Shanghai, China). First, 1/4 of the volume of loading buffer was added to the protein, and the samples were denatured in boiling water for 5 min and aliquoted and stored at −80 °C to avoid repeated freezing and thawing. The proteins were separated by electrophoresis using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 80 V for 30 min and 120 V for 60 min and transferred onto a polyvinylidene difluoride membrane at 280 mA for 75 min. The membranes were blocked with 5% skim milk at the room temperature for 2 h. Membranes were incubated overnight at 4 °C with primary antibodies against NOX2 and NOX4. The membranes were washed three times with Tris-buffered saline Tween for 10 min and incubated for 1 h at the room temperature with appropriate HRP-conjugated reporter antibodies. After three 10-min washes, a ChemiDoc™ Touch Gel imaging system (Bio-Rad, Hercules, CA, USA) was used to visualize immunoreactivity with a chemiluminescent HRP substrate (Vazyme, Nanjing, Jiangsu, China). The band intensities were determined using Image Lab software and expressed relative to GAPDH. All antibodies were purchased from Abcam (Cambridge, MA, USA).
Histopathological examination
Pieces of renal tissue were fixed overnight in 10% formalin solution, dehydrated in ethanol, embedded in paraffin, and then sectioned. The sections were stained with periodic acid-Schiff (PAS) for histological and morphometric analysis. Each sample slice was observed under a microscope (Olympus, Tokyo, Japan) at a magnification of ×400.
Structural glomerular injury was analyzed by the glomerular sclerosis index (GSI). The severity of sclerosis for each glomerulus was graded from 0 to 4+ based on the degree of glomerulosclerosis and mesangial matrix expansion. No lesion was graded as 0, sclerosis values of <25%, 25–50%, >50–75%, and >75% of the glomerulus were graded as 1, 2, 3, and 4, respectively. The GSI was obtained by averaging the scores from counted glomeruli [24].
Immunohistochemical analysis
Paraffin-embedded kidney sections were deparaffinized, hydrated in ethyl alcohol, blocked with blocking solution`for 15 min at the room temperature, and then incubated overnight at 4 °C with rabbit anti-nephrin (1:100; Abcam, Cambridge, MA, USA). Sections were incubated with HRP polymer anti-rabbit IgG at room temperature for 30 min. The slides were then examined under a light microscope at ×400 magnification. Brown areas were considered positive.
Transmission electron microscopy
Kidney tissue was cut (1 mm3) in electron microscope fixative solution, dehydrated in graded acetone and ethanol series, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate. The ultrastructure of podocytes was then observed by transmission electron microscopy.
TUNEL assay
TUNEL assays for renal cell apoptosis were performed on 0.5-μm sections of paraffin-embedded tissue using the Roche TUNEL kit. After conventional dewaxing and sealing treatment, the sections were incubated with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase and digoxigenin-11-dUTP for 1 h, rinsed in PBS three times, and incubated with ovalbumin-labeled horseradish peroxidase for 30 min. The sections were stained with DAB, and the nuclei were counterstained with hematoxylin buffer. The nuclei were stained brown in TUNEL-positive cells. Thirty glomerular sections were observed in each kidney, and the number of positive cells was calculated and averaged.
Statistical analysis
Statistical analysis was conducted using one-way ANOVA followed by the LSD test. The results are expressed as the means ± SE. All statistical analyses were performed using SPSS software version 20. P < 0.05 was considered statistically significant.
Results
Blood pressure
The diagnostic criteria for hypertension in rats are SBP ≥ 150 mmHg and/or DBP ≥ 100 mmHg [25]. As shown in Fig. 1a, b, SBP and DBP were measured at week 4, week 8, and week 12. There was no significant difference in BP among the groups during the treatment period. These results suggest that Hcy and FA had no effect on the BP of SHRs.
The levels of systolic blood pressure (SBP), diastolic blood pressure (DBP), plasma homocysteine (Hcy), serum superoxide dismutase (SOD) and serum malondialdehyde (MDA) in control, hyperhomocysteinemia (HHcy), HHcy + low-dose folic acid (LFA) and HHcy + high-dose folic acid (HFA) rats. a The levels of SBP at week 4, week 8, and week 12, b the levels of DBP at week 4, week 8, and week 12, c the levels of Hcy at week 4, week 8, and week 12, d serum SOD concentrations, and e serum MDA concentrations. *P < 0.05 vs control, #P < 0.05 vs HHcy (n = 8)
Plasma Hcy content
SHRs developed HHcy after intraperitoneal injection of DL-Hcy for 4 weeks. These rats had much higher levels of plasma Hcy (25.60 ± 0.70, 26.60 ± 0.77, and 25.50 ± 0.81 μmol/L in the HHcy, HHcy + LFA and HHcy + HFA groups, respectively) than the levels in the control group (7.33 ± 0.74 μmol/l). The HHcy, HHcy + LFA, and HHcy + HFA groups were then given intraperitoneal injections of DL-Hcy for 8 weeks, and different doses of FA were given to the HHcy + LFA group and HHcy + HFA group. Both HFA and LFA significantly reduced the plasma Hcy level (at the 8th week, 26.54 ± 0.69, 13.74 ± 0.83, and 12.51 ± 0.84 μmol/l in the HHcy, HHcy + LFA and HHcy + HFA groups; at the 12th week, 27.75 ± 0.59, 12.90 ± 0.93, and 10.93 ± 0.83 μmol/l in the HHcy, HHcy + LFA, and HHcy + HFA groups, respectively). However, there was no significant difference in the plasma Hcy level between the HHcy + LFA and HHcy + HFA groups (Fig. 1c).
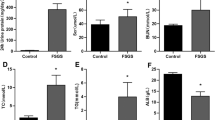
Serum SOD and MDA
Figure 1d, e shows the effects of HHcy and FA on the levels of serum SOD and MDA in all groups. A significant decrease in the serum SOD level was observed in HHcy rats (361.44 ± 11.74 U/ml) compared with control rats (432.01 ± 7.55 U/ml), and both LFA (462.6 ± 8.32 U/ml) and HFA (479.04 ± 7.29 U/ml) counteracted the HHcy-induced reduction in SOD levels. A significant increase was also observed in the serum MDA level in the HHcy group (9.16 ± 0.40 nmol/ml) compared with the control group (7.66 ± 0.29 nmol/ml), and both LFA (6.24 ± 0.36 nmol/ml) and HFA (6.72 ± 0.37 nmol/ml) counteracted the HHcy-induced increase in the MDA level. However, the changes observed in the HHcy + HFA group were insignificant compared with the HHcy + LFA group (P > 0.05).
UACR and GFR
To assess the effects of HHcy and the protective action of FA on the kidneys, the UACR and GFR levels were measured. As shown in Fig. 2a, the UACR was higher in the HHcy group (343.50 ± 6.68 mg/g) than in the control group (263.89 ± 7.37 mg/g). Supplementation with FA significantly reduced the UACR compared with the HHcy group. However, HFA (206.21 ± 3.00 mg/g) did not reduce the UACR to a greater degree than LFA (214.65 ± 7.53 mg/g). Figure 2b shows that GFR significantly decreased in the HHcy group (6.04 ± 0.36 ml/g. day) compared with the control group (8.15 ± 0.43 ml/g. day), and GFR increased in both the HHcy + HFA (8.06 ± 0.44 ml/g. day) and HHcy + LFA (7.64 ± 0.43 ml/g. day) groups, but there was no significant difference between the two groups. These results suggested that LFA treatment significantly reduced HHcy-induced increases in UACR and increased the HHcy-induced decrease in GFR, indicating a protective function against HHcy-induced kidney injury.
Changes in renal function and morphology among control, HHcy, HHcy + LFA, and HHcy + HFA rats. a The levels of urinary albumin creatinine ratio (UACR), b the levels of the glomerular filtration rate (GFR), c the glomerular sclerosis index, *P < 0.05 vs control, #P < 0.05 vs HHcy (n = 8), and d representative images of periodic acid-Schiff (PAS) staining showing glomerular injury (original magnification, ×400)
Histopathological examination
Figure 2c shows that GSI significantly increased in the HHcy group (1.75 ± 0.10) compared with the control group (1.06 ± 0.07), and GSI decreased in both the HHcy + HFA (1.16 ± 0.06) and HHcy + LFA (1.20 ± 0.07) groups, but there was no significant difference between high and low doses of FA. Representative images of PAS staining showing glomerular injury are shown in Fig. 2d. The glomerular capillary loops were wrinkled, and the glomerular balloon spaces widened in the control group (hypertensive). Compared with the control group, the glomerular capillary loops in the HHcy group were significantly wrinkled and thickened, and the glomerular extracellular matrix was increased. Notably, the mesangial matrix increases in glomeruli in LFA and HFA rats could be alleviated compared with HHcy rats.
Expression levels of NOX2 and NOX4 in renal tissues
To examine the expression of NOX2 and NOX4 in the renal cortex, qRT-PCR and western blot analysis were performed. qRT-PCR indicated increased mRNA levels of NOX2 and NOX4 in the glomeruli of SHRs with HHcy compared with control SHRs. Both LFA and HFA inhibited the HHcy-induced expression of NOX2 and NOX4 in glomeruli (Fig. 3a, b). Western blot analysis demonstrated that NOX2 and NOX4 protein levels were significantly higher in the HHcy group than in the control group and that this effect was reversed by treatment with both HFA and LFA (Fig. 3c, d). Furthermore, NOX2 expression was significantly lower in the HHcy + HFA group than in the HHcy + LFA group, although there was no significant difference in Hcy between the two groups (Fig. 3a, c). Oxidative stress parameters in blood and tissues suggested that LFA can alleviate the HHcy-induced oxidative stress response and that FA may have an antioxidant effect independent of Hcy.
The mRNA and protein expression levels of NOX2 and NOX4 in renal tissues. a Relative mRNA levels of NOX2 by quantitative real-time polymerase chain reaction (qRT-PCR) analysis in renal tissues among control, HHcy, HHcy + LFA and HHcy + HFA rats. b Relative mRNA levels of NOX4 by qRT-PCR analysis. c Western blot analyses of NOX2 protein levels in renal tissues, including representative blot images and a densitometric summary of the blot analysis after normalization to GAPDH. d Western blot analyses of NOX4 protein levels in renal tissues, including representative blot images and a densitometric summary of the blot analysis after normalization to GAPDH. *P < 0.05 vs control, #P < 0.05 vs HHcy, †P < 0.05 vs HHcy + LFA (n = 8)
Expression levels of nephrin in renal tissues
qRT-PCR demonstrated that nephrin mRNA levels were significantly downregulated in SHRs with HHcy compared with control rats and that this effect was reversed by treatment with FA (Fig. 4a). However, there was no significant difference in nephrin mRNA levels between the HHcy + LFA and HHcy + HFA groups. Immunohistochemistry showed that nephrin protein expression in the kidneys was significantly lower in the HHcy group than in the control group. However, both LFA and HFA treatment improved nephrin protein expression (Fig. 4b).
The mRNA and protein expression levels of nephrin in renal tissues and the foot processes of podocytes under transmission electron microscopy (TEM) among control, HHcy, HHcy + LFA and HHcy + HFA rats. a Relative mRNA levels of nephrin by qRT-PCR analysis in renal tissues, *P < 0.05 vs control, #P < 0.05 vs HHcy (n = 8). b Representative immunostaining for nephrin in the glomeruli from different groups of rats (original magnification, ×400) and morphological changes in the foot processes of podocytes under TEM
Changes in podocytes observed by TEM
TEM experiments were conducted to evaluate the ultrastructural changes in podocytes. As shown in Fig. 4b, the glomerular basement membrane of the control group (hypertensive) was thickened, and partial fusion of podocyte foot processes was observed. However, compared with the control group, the brush-like structure of the podocyte foot process disappeared and was widely fused in the HHcy group, while the ultrastructure of the podocyte was significantly improved in the HHcy + LFA and HHcy + HFA groups, and this effect was greater with HFA.
TUNEL assay
As shown in Fig. 5a, b, the number of TUNEL-positive cells in kidney tissue was much higher in the HHcy group (3.88 ± 0.17) than in the control group (2.09 ± 0.15). The number of TUNEL-positive podocytes was significantly decreased after LFA and HFA treatment (2.31 ± 0.18 in HHcy + LFA and 2.03 ± 0.16 in HHcy + HFA rats) (p < 0.05) compared with the HHcy group, and there was no significant difference between the FA doses. The TUNEL assay results suggest that HHcy increased podocyte apoptosis in SHRs and that LFA alleviated HHcy-induced apoptosis.
Podocyte apoptosis by TUNEL assay among control, HHcy, HHcy + LFA and HHcy + HFA rats. a Representative images of podocyte apoptosis in the glomeruli from different groups of rats (original magnification, ×400), where the nuclei are stained brown in TUNEL-positive cells. b The number of TUNEL-positive cells/glomeruli. *P < 0.05 vs control, #P < 0.05 vs HHcy (n = 8)
Discussion
In the present study, we demonstrated that lowering the plasma Hcy level by LFA treatment attenuates HHcy-induced glomerular injury in SHRs. Consistent with a previous report, elevated plasma Hcy was associated with glomerular damage in Dahl salt-sensitive hypertensive rats [26]. In the present study, considering the high incidence of HHcy in China, SHRs were injected intraperitoneally with DL-Hcy for 12 weeks to create an animal model for investigating the mechanism of the effect of HHcy on renal damage in hypertension, which has high clinical relevance. Previous research showed that enalapril–FA therapy slows the progression of CKD in patients with hypertension with mild-to-moderate CKD to a greater degree than enalapril alone [27]. However, the FA dose used for HHcy intervention varies significantly across previous studies [20, 22, 28], and different doses of FA have produced inconsistent results. To clarify the effects of different doses of FA on Hcy and related renal damage, we established LFA and HFA groups. Our results showed that both doses significantly reduced the plasma Hcy level, with no significant difference between the two groups. The results of this study are consistent with the results of Tamadon et al. [29], which showed that LFA can effectively reduce plasma Hcy levels. In addition, our results showed that Hcy had no effect on the BP of SHRs, suggesting that Hcy-associated renal damage may be independent of BP. We therefore turned our attention to the mechanism underlying the effects of Hcy and FA on renal damage in hypertension.
Research has shown that HHcy is associated with changes in the structure and function of cerebral blood vessels, and oxidative stress appears to play a major role in mediating these changes [29]. Yi et al. [30] found that Hcy activates NADH/Nox via ceramide-stimulated Rac GTPase activity in rat mesangial cells. A previous study has shown that excessive activation of Nox is an important cause of excessive reactive oxygen species production in cells [31]. In the current study, HHcy induced a decrease in serum SOD and an increase in serum MDA levels, and these effects were counteracted by FA treatment. We also observed that NOX2 and NOX4 mRNA and protein levels in kidney tissue were significantly increased in the HHcy group compared with the control group, and this effect was also counteracted by FA treatment. The above results indicate that hypertensive rats with HHcy are in a state of oxidative stress and that LFA and HFA can ameliorate this condition by reducing Hcy. In addition, although there was no significant difference in the plasma Hcy level between the HHcy + HFA and HHcy + LFA groups, NOX2 expression was lower in the HHcy + HFA group. This suggested that FA may play an antioxidant role independent of Hcy by inhibiting NOX2 expression. FA has been reported to exert an independent antioxidant effect by inhibiting Nox-mediated superoxide anion production in human kidney proximal tubular cells [32], and this effect has also been demonstrated in the heart and liver.
UACR is an important warning sign of early renal damage, especially glomerular filtration barrier damage. We found that UACR significantly increased and that GFR significantly decreased in the HHcy group. Furthermore, compared with the control group, the glomerular capillary loops in the HHcy group were significantly wrinkled and thickened, the glomerular extracellular matrix and the GSI were increased, and the podocyte foot process was widely fused, which was consistent with functional alterations in the kidney. These changes in glomerular function and structure were associated with a significantly decreased level of nephrin, which is an important podocyte slit diaphragm molecule that plays a critical role in maintaining the glomerular filtration barrier [33, 34]. In SHRs supplemented with LFA and HFA, nephrin expression was increased, and glomerular structure and function were significantly improved. Compared with the HHcy + LFA group, the expression of nephrin in the HHcy + HFA group was slightly higher, and podocyte ultrastructure, UACR and GFR were slightly improved. This may have been related to the decrease in NOX2 and the independent antioxidant effect of FA, but there were no significant differences in the above parameters between the two groups. Recent research [35] has also demonstrated that HHcy decreases nephrin expression in gp91+/+ (NOX2) mice but not gp91−/− mice. Considering the relevant literature, the results of this study showed that Hcy increased oxidative stress and downregulated nephrin expression, aggravating the damage of podocytes and glomerular structure and renal function in hypertensive rats, and LFA improved renal damage by reducing Hcy and playing an antioxidant role.
Nephrin is not only a structural molecule of the slit diaphragm but also an important signal transduction molecule that affects podocyte survival [36]. Huber et al. found that phosphorylation of intracellular nephrin can initiate PI3K/AKT-dependent signal transduction in glomerular podocytes and inhibit purinomycin-induced podocyte apoptosis [37]. Our data also suggest that HHcy reduces the expression of nephrin in podocytes by enhancing oxidative stress, which leads to podocyte apoptosis, and that LFA may increase nephrin expression and inhibit podocyte apoptosis by reducing Hcy and exerting antioxidant effects. In addition, Huber et al. also found that tyrosine residue phosphorylation in the intracellular segment of nephrin can further activate the downstream p38MAPK signaling pathway and enhance the activity of the transcription factor AP-1 [38], which, as a key transcription factor regulating cell survival and death pathways, is involved in the regulation of cell apoptosis [39]. Therefore, HHcy reduces the expression of nephrin and induces apoptosis of podocytes, which may involve the PI3K/AKT and p38MAPK signaling pathways mediated by nephrin.
In conclusion, based on a hypertensive rat model with HHcy, the current study established that HHcy synergistically increased glomerular function and morphological damage in hypertensive rats. These pathogenic effects of Hcy were independent of BP and were related to increased oxidative stress and downregulated nephrin expression, ultimately leading to podocyte apoptosis. By reducing plasma Hcy levels and exerting antioxidant effects, LFA delayed the development of HHcy-induced glomerular damage associated with hypertension, indicating the potential efficacy of LFA in treating renal damage in hypertensive patients with HHcy. However, the signaling mechanism of HHcy-induced podocyte injury remains to be further studied.
References
Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617.
Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. Jama. 2015;313:1325–35.
Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, et al. Plasma homocysteine as a risk factor for Vascular Disease. Jama. 1997;277:1775–81.
Jiang S, Li J, Zhang Y, Venners SA, Tang G, Wang Y, et al. Methylenetetrahydrofolate reductase C677T polymorphism, hypertension and risk of stroke: a prospective, nested case-control study. The. Int J Neurosci. 2017;127:253–60.
Towfighi A, Markovic D, Ovbiagele B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: a nationwide study. J Neurol Sci. 2010;298:153–7.
Yun L, Xu R, Li G, Yao Y, Li J, Cong D, et al. Homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR are risk factors of early renal damage in hypertension in a Chinese Han population. Medicine 2015;94:e2389.
Kuang ZM, Wang Y, Feng SJ, Jiang L, Cheng WL. Association between plasma homocysteine and microalbuminuria in untreated patients with essential hypertension: a case-control study. Kidney Blood Press Res. 2017;42:1303–11.
Chuong Nguyen MV, Lardy B, Paclet M-H, Rousset F, Berthier S, Baillet A, et al. Les NADPH oxydases, Nox. Médecine/Science. 2015;31:43–52.
Cifuentes-Pagano E, Csanyi G, Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci. 2012;69:2315–25.
Gorin Y, Block K. Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free radical biology &. Medicine 2013;61:130–42.
Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci 2013;125:361–82.
Shastry S, Ingram AJ, Scholey JW, James LR. Homocysteine induces mesangial cell apoptosis via activation of p38-mitogen-activated protein kinase. Kidney Int 2007;71:304–11.
Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, et al. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int 2006;70:88–96.
Ngarashi D, Fujikawa K, Ferdaus MZ, Zahid HM, Ohara H, Nabika T. Dual inhibition of NADPH oxidases and xanthine oxidase potently prevents salt-induced stroke in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2019;42:981–9.
Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, et al. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem. 2010;285:25285–95.
Deen WM. What determines glomerular capillary permeability? J Clin Investig. 2004;114:1412–4.
Li M, Chen J, Li YS, Feng YB, Gu X, Shi CZ. Folic acid reduces adhesion molecules VCAM-1 expession in aortic of rats with hyperhomocysteinemia. Int J Cardiol. 2006;106:285–8.
Romecin P, Atucha NM, Navarro EG, Ortiz MC, Iyu D, Rosado JA, et al. Role of homocysteine and folic acid on the altered calcium homeostasis of platelets from rats with biliary cirrhosis. Platelets 2017;28:698–705.
Li Y, Qin X, Luo L, Wang B, Huo Y, Hou FF, et al. Folic acid therapy reduces the risk of mortality associated with heavy proteinuria among hypertensive patients. J Hypertens. 2017;35:1302–9.
Cagnacci A, Cannoletta M, Volpe A. High-dose short-term folate administration modifies ambulatory blood pressure in postmenopausal women. A placebo-controlled study. Eur J Clin Nutr. 2009;63:1266–8.
Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults. Nutr J. 2012;11:2.
Zappacosta B, Mastroiacovo P, Persichilli S, Pounis G, Ruggeri S, Minucci A, et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients 2013;5:1531–43.
Zhiqing W, Jing W, Haili X, Shaozhuang L, Chunxiao H, Haifeng H, et al. Renal function is ameliorated in a diabetic nephropathy rat model through a duodenal-jejunal bypass. Diabetes Res Clin Pract. 2014;103:26–34.
Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 1984;26:137–43.
Yu B, Xu D, Sun H, Yang K, Luo M. Comparative analysis of mechanical properties of PWV, NO and ascending aorta between WHY Rats and SHR Rats. Acta Cardiol Sin. 2015;31:6.
Li N, Chen Y-F, Zou A-P. Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension. Hypertension 2002;39:443–8.
Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China stroke primary prevention trial. JAMA Intern Med. 2016;176:1443–50.
Tamadon MR, Jamshidi L, Soliemani A, Ghorbani R, Malek F, Malek M. Effect of different doses of folic acid on serum homocysteine level in patients on hemodialysis. Iran J Kidney Dis. 2011;5:93–96.
Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke 2004;35:345–7.
Yi F, Zhang A, Janscha J, Li P, Zou A. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int 2004;66:1977–87.
Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010;88:241–8.
Hwang SY, Siow YL, Au-Yeung KK, House J, O K, et al. Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am J Physiol Ren Physiol. 2011;300:F189–98.
Wartiovaara J, Öfverstedt L-G, Khoshnoodi J, Zhang J, Mäkelä E, Sandin S, et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Investig. 2004;114:1475–83.
Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10:543–9.
Zhang C, Hu JJ, Xia M, Boini KM, Brimson CA, Laperle LA, et al. Protection of podocytes from hyperhomocysteinemia-induced injury by deletion of the gp91phox gene. Free Radic Biol Med. 2010;48:1109–17.
Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–52.
Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–28.
Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276:41543–6.
Wang H, Song W, Hu T, Zhang N, Miao S, Zong S, et al. Fank1 interacts with Jab1 and regulates cell apoptosis via the AP-1 pathway. Cell Mol life Sci. 2011;68:2129–39.
Acknowledgements
This study was supported by the Shandong Provincial Key Research and Development Programme Foundation, China (Grant No. 2018GSF118009), the Technology Programme Foundation of Jinan, China (Grant No. 201821007), and the Shandong Provincial Medical Science and Technology Development Programme Foundation, China (Grant No. 2017WS462).
Author information
Authors and Affiliations
Contributions
NG and RX designed the experiment; NG, YZZ, LLi, PC, and LLin performed the experiments; LLei, and XZ analyzed the data and prepared the figures; NG, YZZ, and RX wrote the manuscript; and RX had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, N., Zhang, Y., Lei, L. et al. Low doses of folic acid can reduce hyperhomocysteinemia-induced glomerular injury in spontaneously hypertensive rats. Hypertens Res 43, 1182–1191 (2020). https://doi.org/10.1038/s41440-020-0471-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0471-8
Keywords
This article is cited by
-
Binge drinking as a potential cardiometabolic risk factor during adolescence in rats: novel protective antinitrosative mechanism of folic acid via caveolin-1
Hypertension Research (2025)
-
Homocysteine level is positively and independently associated with serum creatinine and urea nitrogen levels in old male patients with hypertension
Scientific Reports (2020)