Abstract
The growing recognition of the importance of interpregnancy weight management in reducing hypertensive disorders of pregnancy (HDP) underscores the importance of effective preventive strategies. However, developing effective systems remains a challenge. We aimed to bridge this gap by constructing a prediction model. This study retrospectively analyzed the data of 1746 women who underwent two childbirths across 14 medical facilities, including both tertiary and primary facilities. Data from 2009 to 2019 were used to create a derivation cohort (n = 1746). A separate temporal-validation cohort was constructed by adding data between 2020 and 2024 (n = 365). Furthermore, the external-validation cohort was constructed using the data from another tertiary center between 2017 and 2023 (n = 340). We constructed a prediction model for HDP development in the second pregnancy by applying logistic regression analysis using 5 primary clinical information: maternal age, pre-pregnancy body mass index, and HDP history; and pregnancy interval and weight change velocity between pregnancies. Model performance was assessed across all three cohorts. HDP in the second pregnancy occurred 7.3% in the derivation, 10.1% in the temporal-validation, and 7.9% in the external-validation cohorts. This model demonstrated strong discrimination, with c-statistics of 0.86, 0.88, and 0.86 for the respective cohorts. Precision-recall area under the curve values were 0.90, 0.85, and 0.91, respectively. Calibration showed favorable intercepts (−0.02 to −0.00) and slopes (0.96–1.02) for all cohorts. In conclusion, this externally validated model offers a robust basis for personalized interpregnancy weight management goals for women planning future pregnancies.

Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP), with an incidence of 8–10%, is a major cause of maternal mortality, and because there is no effective treatment, prevention is crucial [1]. The implementation of HDP prevention measures can be categorized into two phases: pre- and post-conception. Among post-conception measures, low-dose aspirin is recognized as an effective prophylactic intervention along with gestational weight gain management [2, 3]. Whereas weight control is crucial as a pre-conception measure [4, 5].
Interpregnancy care (IPC) has been endorsed as a pragmatic pre-conception strategy for HDP prevention [4, 5]. The prevailing recommendation for interpregnancy weight management, as a part of IPC, is simply to attain a body mass index (BMI) of 18.5–25.0 kg/m2 for prevention of HDP in the subsequent pregnancy [4, 5]. This target may be unattainable for severely obese women who arguably stand to benefit the most from the intervention. Thus, we previously proposed the concept of “annual BMI changes”, defined as the annual pace of pre-pregnancy BMI fluctuations from the previous to the subsequent pregnancy, as an alternative framework allowing for achievable goal setting for all [6,7,8]. Suppressing annual BMI change below 0.6 kg/m2/year was found to minimize the risk “elevation” of HDP development in the subsequent pregnancy [6], which was considered useful for population approach from a preventive medicine perspective [9].
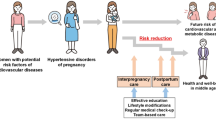
As for high-risk approach aiming for risk “reduction”, precision medicine is considered ideal [9, 10], because weight reduction requires their own active involvement [11]. It is known that promoting patient behavior change and posits that behavior modification is facilitated by understanding one’s own risk, and anticipated outcomes of action or inaction [12]. With using a prediction model, visualizing the current risk and the degree of risk modification can be achieved through their own efforts. As shown in Fig. 1, our proposing bidirectional communication-based prevention strategy initiates with a risk assessment, evaluating parameters from the previous pregnancy (Step 1: risk assessment). Subsequently, the model inputs the period until the next pregnancy and sets a timeframe for weight management (Step 2: family planning). Finally, prediction model utilizes these parameters to visually represent how modifications in annual BMI change can alter the probability of HDP risk in future pregnancies, with the age-related BMI gain (0.2 kg/m²/year) [11, 13, 14] serving as a reference (Step 3: goal setting). A suitable prediction model is required to implement this strategy, but currently not available.
Conceptual Diagram of Bidirectional Communication-Based Interpregnancy Weight Management. Based on the clinical information derived from the risk assessment (step 1) and family planning (step 2), the prediction model estimated the risk of HDP in the next pregnancy corresponding to annual BMI changes (step 3). BMI body mass index, HDP hypertensive disorders of pregnancy
Previously, some prediction models for HDP have been proposed, but they were all designed to evaluate and intervene during the post-conception phase [15, 16]. Notably, no models are specifically aimed at pre-conception measures, including inter-conception weight management. Thus, the objective of this study was to develop and validate a prediction model for interpregnancy weight management aimed at reducing the risk of HDP in future pregnancies.
Methods
Derivation and validation Cohort
We used the dataset from our previous study [6] as the derivation cohort. This dataset consisted of the electronic medical records of women aged ≥15 years with singleton pregnancies who had two deliveries at 2 tertiary facilities (Nagoya University Hospital and TOYOTA Memorial Hospital, Aichi Prefecture) and 12 private obstetric facilities (Kishokai Medical Corporation, Aichi and Gifu Prefecture) between 2009 and 2019 (Fig. 2). We defined the first and second pregnancies during the study period as the index and subsequent pregnancies, respectively. The exclusion criteria were stillbirth before 22 weeks of gestation, chronic hypertension, and missing data for maternal blood pressure and pre-pregnancy BMI. Using the same inclusion and exclusion criteria, data from the two tertiary centers between January 2020 and June 2024 were retrospectively added. Women with singleton pregnancies who delivered a subsequent pregnancy during this period were included in the temporal-validation cohort. There were no overlapping individuals in both cohorts: they were independent. Furthermore, the external-validation cohort was constructed using data from another tertiary center, Ogaki municipal hospital in Gifu prefecture, covering the period from January 2017 to December 2023.
Flowchart Showing the Composition of the Derivation, Temoral- and External-Validation Cohorts. This image presents a schematic outline of the selection of the study cohorts. Each case in the study is depicted by a sequence of symbols: a circle (●) for the initial or index pregnancy and a diamond (◆) for the subsequent pregnancy. The derivation cohort comprised cases from 12 primary facilities and 2 tertiary centers with subsequent pregnancies that resulted in deliveries between 2009 and 2019, represented by blue lines. The temporal-validation cohort consisted of cases exclusively from 2 tertiary centers with subsequent pregnancies delivered between 2020 and 2024, as shown by the red lines. The external-validation cohort consisted of cases from another tertiary center with subsequent pregnancies that resulted in deliveries between 2017 and 2023, as shown by the yellow lines. BMI body mass index
This study was approved by the ethics board of Nagoya University Hospital (approval number: 2015–0415), and the study was conducted following the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study. We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines.
Outcome
The primary outcome was the development of HDP during the subsequent pregnancy. Our primary outcome, HDP, comprised gestational hypertension and preeclampsia, that is, the development of hypertension after 20 weeks of gestation. HDP was reassessed by two obstetric specialist (ST) and (MN) who reviewed the medical records of all participants with using the same current diagnostic criteria [17]. Although chronic hypertension is a subtype of HDP [17], because the primary outcome was the onset of the HDP, it was placed in the exclusion criteria.
Definitions of variables
We used self-reported maternal pre-pregnancy body weight obtained during routine practice and calculated the pre-pregnancy BMI (kg/m2). ΔBMI was defined as a change in pre-pregnancy BMI from the index pregnancy to the subsequent pregnancy, as previously reported [18, 19]. The pregnancy interval was defined as the interval from the expected date of delivery (EDD) of the index pregnancy to that of the subsequent pregnancy. EDD was determined based on the last menstrual period or measurement of the crown-rump length during routine practice. The annual BMI change (kg/m2/year) was calculated as the ΔBMI/pregnancy interval [6,7,8].
Developing a prediction model
Using data from the derivation cohort, we constructed a prediction model for the probability of HDP during subsequent pregnancies (predicted probability). This model was calculated using a logistic regression equation [20] with our previously reported optimal five predictors: maternal age and pre-pregnancy BMI at the index pregnancy (Age and BMI, respectively), presence (1) or absence (0) of HDP at the index pregnancy (HDPind), pregnancy interval (Pi), and annual BMI change (ABc) [6].
Statistical analysis
The differences in characteristics among the three cohorts were statistically analyzed using one-way analysis of variance (ANOVA). Dunnett’s test post hoc ANOVA test was used to identify significant differences between derivation and two validation cohorts. Chi-square tests were used for categorical variables. Statistical significance was set at two-tailed p-value of <0.05.
Model performance was evaluated across all three cohorts with respect to discrimination, calibration, and clinical usefulness. Discrimination was evaluated by estimating the area under the receiver operating characteristic curve (c-statistic) [21] and performing precision-recall curve analysis [22]. Calibration was evaluated by plotting flexible calibration curves based on locally estimated scatterplot smoothing (LOESS) [23]. The clinical usefulness of the model was evaluated using decision curve analysis, which determines the net benefit across a range of threshold probabilities [24]. The net benefit was calculated as the number of true positives (TPs) minus the number of false positives (FPs), with the latter weighted according to the threshold probability. Plots were generated to compare the net benefit of the three scenarios: one assuming all patients were at high risk, another assuming no patients were at high risk, and one based on the logistic regression prediction model [24]. Statistical analyses were performed using SPSS ver. 28.0 (IBM, Inc.) and R ver.4.1.3 (R Foundation for Statistical Computing).
Results
Baseline characteristics and outcomes of derivation and validation Cohorts
The derivation, temporal-validation, and external-validation cohorts included 1746, 365, and 340 individuals, respectively (Table 1). No data regarding outcomes or predictors were missing. HDP in the subsequent pregnancy occurred 7.3% (128/1,746) in the derivation, 10.1% (37/365) in the temporal-validation, and 7.9% (27/340) in the external-validation cohorts. Concerning the index pregnancy parameters, pre-pregnancy BMI was higher in both the temporal-validation (22.1 ± 4.0 kg/m²) and external-validation cohorts (22.3 ± 4.8 kg/m²) compared to the derivation cohort (20.9 ± 3.3 kg/m²) (p < 0.001). There were also significant differences in the prevalence of overweight ( ≥ 23.0 kg/m²) individuals, with the highest prevalence in the temporal-validation cohort (41.1%), followed by the external-validation cohort (34.1%) and the derivation cohort (16.8%) (p < 0.001). Regarding the interpregnancy parameters, the pregnancy interval was significantly longer in the temporal-validation (3.2 ± 2.2 years) and external-validation cohorts (2.5 ± 1.1 years) compared to the derivation cohort (2.3 ± 0.9 years) (p < 0.001). In the subsequent pregnancy parameters, the rate of HDP in the subsequent pregnancy did not differ significantly among the three cohorts (p = 0.192).
Model performance
The c-statistics for the derivation, temporal-validation, and external-validation cohorts were 0.86 (95%CI 0.82–0.89), 0.88 (95%CI 0.81–0.93), and 0.86 (95%CI 0.78–0.93), respectively (Fig. 3A). Precision recall analyses also showed that the area under the curve was 0.90 (95%CI 0.89–0.94), 0.85 (95%CI 0.82–0.92), and 0.91 (95%CI 0.86–0.95) for the respective cohorts. (Fig. 3B). These results indicate that the individuals in each cohort could be strongly discriminated by the prediction model. Calibration analyses revealed high slopes and low intercepts (Fig. 3C–E). Specifically, the derivation cohort exhibited an intercept of −0.00 (95%CI −0.01–0.01) and a slope of 1.02 (95%CI 0.97–1.07). Similarly, the temporal- and external-validation cohorts presented intercepts of −0.02 (95%CI −0.04–0.00) with a slopes of 0.97 (95%CI 0.87–1.06), and −0.00 (95%CI −0.04–0.013) with a slopes of 0.96 (95%CI 0.82–1.11), respectively. Notably, the ideal line (dashed line) fell within the 95%CI of the all calibration curves, indicating a well-calibrated model.
Model Performance. A Receiver Operating Characteristic (ROC) Curves. The graph presents the ROC curves for the derivation cohort (blue) and temporal- (red) and external- (yellow) validation cohorts; the x-axis indicates the false-positive rate (FPR), and the y-axis shows the true-positive rate (TPR). ROC, receiver operating characteristic; 95% CI, 95% confidence interval. B Precision-Recall Curves for the Derivation and Validation Cohorts. The plot delineates the precision-recall relationship for the derivation cohort (blue) and temporal- (red) and external- (yellow) validation cohorts. The x-axis represents recall, and the y-axis indicates precision. C–E Calibration Analyses. These plots illustrate the calibration of the prediction model for the derivation (C) and temporal- (D) external- (E) and validation cohorts, by juxtaposing the mean predicted probability (x-axis) with the mean actual outcome (y-axis). The loess-smoothed line represents the nonlinear relationship between these two variables, and the shaded region indicates the 95% confidence interval (CI) around the smoothed estimates. An ideal prediction model would have all its data points, and the smoothed line would adhere closely to the dashed 45-degree reference line, indicating signaling-impeccable calibration. F–H Decision Curve Analyses. Decision curve analysis for the derivation (E), temporal- (F), and external- (H) validation cohorts. These graphs compare the net benefits derived from the three risk-stratification strategies across a range of threshold probabilities. The red line indicates the net benefit of using our predictive model, the green line assumes that all individuals are at high risk, and the blue line indicates that no individual is at high risk
Evaluation of the clinical usefulness and application of the model
Decision curve analysis for the three cohorts (Fig. 3F–H) demonstrated that the ‘Use model’ (red line) consistently surpasses the ‘Assume all individuals are high risk’ (green line) and ‘Assume no individuals are high risk’ (blue line) across the threshold probabilities. This indicates that our model provides a greater net benefit compared to the extreme strategies of treating everyone or treating no one.
Discussion
Main findings
Our study successfully constructed and validated a logistic regression-based interpregnancy weight management-focused prediction model that exhibited high accuracy in forecasting HDP in subsequent pregnancies. The existing prediction models are designed for assessment at the post-conception stage [15, 16, 25], and this is the first prediction model for interpregnancy weight management, a type of pre-conception care. Unlike typical supervised learning assumptions that expect similar characteristics across derivation and validation cohorts [26], our model maintained reliability even with divergent cohort characteristics, underscoring its robustness and generality. Remarkably, this highly accurate prediction model comprises a simplified set of five key variables, which greatly enhances its practicality in clinical use.
Interpretation
“Interpretability” and “Accuracy” are important in constructing a prediction model [27]. “Interpretability” is a passive property of a model, which indicates the degree to which a given model can be interpreted by a human observer. “Accuracy” refers to the degree of the model performance. Interpretability and accuracy generally have a trade-off relationship [28]. Logistic regression, a conventional method, has higher interpretability and lower accuracy, whereas machine learning has lower interpretability and higher accuracy [27].
It is widely recognized that individuals are reluctant to adopt tools that cannot be directly interpreted. Thus, there are concerns about the abundance of research that emphasizes new algorithms without focusing on user-friendliness, practical interpretability, or efficacy for end users [29, 30]. Furthermore, when implementing machine learning-based prediction models in clinical settings, the operational environment of programming languages used in model construction, such as R or Python, is necessary. In contrast, prediction models developed using logistic regression analysis can be replicated using any software capable of logarithmic calculations. Therefore, the results of the present study, obtained with reasonable accuracy using logistic regression analysis, are advantageous for clinical applications.
A recent meta-analysis on externally validated prediction models for HDP concluded that models based solely on maternal information exhibit equivalent predictive accuracy to those augmented with various biomarkers [25]. This suggests that maternal information plays a significantly crucial role in predicting the occurrence of HDP. Consequently, the high predictive accuracy achieved in our study using only clinical data aligns logically with these insights. This capability indicates the potential for high-accuracy predictions in low-resource countries and regions.
Implications
Our prediction model makes women aware of their own HDP risks before pregnancy and visualize that one can reduce risk through one’s efforts. Using the outputs, establishing weight management goals through bidirectional communication between healthcare providers and women is beneficial for the implementation of interpregnancy weight management. This interactive process allows the integration of each individual’s risk tolerance and commitment to lifestyle modifications, thereby enabling the setting of personalized and realistic targets. That is, bidirectional communication enables women to consider their past experiences and current environment to ascertain the degree of attainable or appropriate weight loss. Concurrently, healthcare providers can evaluate whether the goals are reasonable.
Strength and limitations
The primary strength of this study is its novel approach, which focuses on pragmatic applications for interpregnancy weight management, a part of IPC. Secondary, the prediction model, which sets weight management objectives based on annual BMI changes, ensures consistency and reliability irrespective of variations in pregnancy intervals. Additionally, the model design, utilizing only five optimal covariates, not only enhances user-friendliness but also reduces the risk of inaccuracy due to missing data in clinical use.
This study had some limitations. First, it was retrospective, and the annual BMI changes were not intervention-induced. Further studies are required to determine whether intervention-induced weight reduction reduces the risk of HDP in subsequent pregnancies. Accordingly, we plan to conduct a prospective study on weight management as a part of IPC using the prediction model developed in this study. Second, self-reported body weight was used to calculate the pre-pregnancy BMI. However, as almost all participants weighed themselves during antenatal checkups in the first trimester of pregnancy, the difference between their self-reported and actual weights was likely to be minimal. Third, all validations in this study were conducted using pregnancy data from within Japan. To guarantee its generalizability, geographic validation is necessary. Nevertheless, the prediction model developed herein utilizes solely clinical information, making it applicable across countries and regions regardless of their economic status.
Perspective of Asia
The global rise in obesity is a growing issue, and Asia is no exception. In particular, Asians are more sensitive to the health risks of obesity compared to Western populations [31]. However, a major problem is that no standardized methods for weight management during IPC have been established [32]. Additionally, cultural, racial, and economic factors have been reported to influence postpartum weight loss programs [32, 33].
This study succeeded in developing a prediction model for setting weight management goals during IPC, using a cohort predominantly composed of Asians. We believe this represents a significant step towards more personalized medicine, in contrast to the uniform goal-setting recommended by conventional guidelines [4, 5]. Moving forward, we plan to conduct further research to establish a practical management system based on this prediction model in Asian population. Our future research will focus on how effective management can improve perinatal outcomes and, ultimately, extend healthy life expectancy.
Conclusion
This interpregnancy weight management-focused prediction model is anticipated to offer personalized risk estimations and a basis for establishing weight management goals intended to decrease the risk of future HDP for women who want to have their next pregnancy.
References
Wu P, Green M, Myers JE. Hypertensive disorders of pregnancy. BMJ. 2023;381:e071653.
Truong YN, Yee LM, Caughey AB, Cheng YW. Weight gain in pregnancy: does the Institute of Medicine have it right? Am J Obstet Gynecol. 2015;212:362.e1–8.
Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:110–20.e6.
American College of N-M, the National Association of Nurse Practitioners in Women’s H, American College of O, Gynecologists, the Society for Maternal-Fetal M, Louis JM, et al. Interpregnancy care. Am J Obstet Gynecol. 2019;220:B2–B18.
Obstetric Care Consensus No. 8 Summary: interpregnancy care. Obstet Gynecol. 2019;133:220–5.
Tano S, Kotani T, Ushida T, Yoshihara M, Imai K, Nakano-Kobayashi T, et al. Annual body mass index gain and risk of hypertensive disorders of pregnancy in a subsequent pregnancy. Sci Rep. 2021;11:22519.
Tano S, Kotani T, Ushida T, Yoshihara M, Imai K, Nakano-Kobayashi T, et al. Annual body mass index gain and risk of gestational diabetes mellitus in a subsequent pregnancy. Front Endocrinol (Lausanne). 2022;13:815390.
Tano S, Kotani T, Ushida T, Yoshihara M, Imai K, Nakano-Kobayashi T, et al. Optimal annual body mass index change for preventing spontaneous preterm birth in a subsequent pregnancy. Sci Rep. 2022;12:17502.
Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–8.
Khoury MJ, Iademarco MF, Riley WT. Precision public health for the era of precision medicine. Am J Preventive Med. 2016;50:398.
Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes (Lond). 2006;30:543–51.
Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–83.
Tanamas SK, Shaw JE, Backholer K, Magliano DJ, Peeters A. Twelve-year weight change, waist circumference change and incident obesity: the Australian diabetes, obesity and lifestyle study. Obes (Silver Spring). 2014;22:1538–45.
Tano S, Kotani T, Ushida T, Iitani Y, Imai K, Kinoshita F, et al. Trend changes in age-related body mass index gain after coronavirus disease 2019 pandemic in Japan: a multicenter retrospective cohort study. Reprod Biol Endocrinol. 2023;21:7.
Poon LCY, Kametas NA, Pandeva I, Valencia C, Nicolaides KH. Mean Arterial Pressure at 11 + 0 to 13 + 6 weeks in the Prediction of Preeclampsia. Hypertension. 2008;51:1027–33.
Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;30:742–9.
Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 international society for the study of hypertension in pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148–69.
Bogaerts A, Van den Bergh BRH, Ameye L, Witters I, Martens E, Timmerman D, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol. 2013;122:999–1009.
Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194:916–20.
Boateng EY, Abaye DA. A review of the logistic regression model with emphasis on medical research. J Data Anal Inf Process. 2019;7:190.
Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015;314:1063–4.
He H, Garcia EA. Learning from imbalanced data. IEEE Trans Knowl Data Eng. 2009;21:1263–84.
Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–76.
Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6.
Tiruneh SA, Thanh Vu TT, Moran LJ, Callander EJ, Allotey J, Thangaratinam S, et al. Externally validated prediction models for pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2024;63:592–604.
Hastie T, Tibshirani R, Friedman JH, Friedman JH The elements of statistical learning: data mining, inference, and prediction. 2. Springer 2009.
Barredo Arrieta A, Díaz-Rodríguez N, Del Ser J, Bennetot A, Tabik S, Barbado A, et al. Explainable Artificial Intelligence (XAI): concepts, taxonomies, opportunities and challenges toward responsible AI. Inf Fusion. 2020;58:82–115.
Baryannis G, Dani S, Antoniou G. Predicting supply chain risks using machine learning: the trade-off between performance and interpretability. Future Gener Comput Syst. 2019;101:993–1004.
Zhu JC, Liapis A, Risi S, Bidarra R, Youngblood GM. Explainable AI for designers: a human-centered perspective on mixed-initiative co-creation. IEEE Conf Comput Int. 2018. <Go to ISI>://WOS:000841411000064.):458–65.
Goodman B, Flaxman S. European union regulations on algorithmic decision making and a “right to explanation. AI Mag. 2017;38:50–7.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Lim S, Liang X, Hill B, Teede H, Moran LJ, O'Reilly S. A systematic review and meta‐analysis of intervention characteristics in postpartum weight management using the TIDieR framework: a summary of evidence to inform implementation. Obes Rev. 2019;20:1045–56.
Setse R, Grogan R, Cooper LA, Strobino D, Powe NR, Nicholson W. Weight loss programs for urban-based, postpartum African-American women: perceived barriers and preferred components. Mater Child Health J. 2008;12:119–27.
Acknowledgements
During the preparation of this work the authors used DeepL and ChatGPT4 for tranlation and academic editing. After using these tools, the authors reviewed and edited the content as needed and takefull responsibility for the content of the publication. We also thank Editage (www.editage.jp) for English language editing.
Funding
ST was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (23K19800) in the decision to submit the article for publication. The funder had no role in study including study design, data collection, analysis, interpretation or writing. The corresponding authors had full access to all the data in this study and had final responsibility for the decision to publish. The Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (23K19800) in the decision to submit the article for publication. Open Access funding provided by Nagoya University.
Author information
Authors and Affiliations
Contributions
S.T., T.K., and M.Yo. conceived the study. S.T. and F.K. performed the statistical analyses. S.T., T.K., T.U., S.M., K.I., T.N.-K., Y.M., M.N., S.Y., M.Ya., Y.K., and H.O. collected and interpreted the clinical data. S.T. and T.K. drafted the manuscript. All authors contributed to the interpretation of the results and the approval of the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Ethical approval was granted prior to study start by the ethics board of Nagoya University Hospital (approval number: 2015–0415) on 4th March 2016, with amendments on 24th June 2021 (22550) and 5th September 2022 (26473).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tano, S., Kotani, T., Ushida, T. et al. Visualizing risk modification of hypertensive disorders of pregnancy: development and validation of prediction model for personalized interpregnancy weight management. Hypertens Res 48, 884–893 (2025). https://doi.org/10.1038/s41440-024-02024-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-02024-8
Keywords
This article is cited by
-
Glucose variability as a key mediator in the relationship between pre-pregnancy overweight/obesity and late-onset hypertensive disorders of pregnancy
Scientific Reports (2025)
-
Predicting gestational diabetes before conception for personalized interpregnancy weight management
Scientific Reports (2025)
-
Emerging evidence-based inter-conception cares for prevention of hypertensive disorders of pregnancy
Hypertension Research (2025)