Abstract
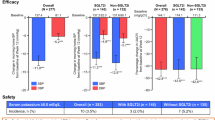
Effective management of blood pressure (BP) and albuminuria are crucial for suppressing chronic kidney disease (CKD) progression and cardiovascular risks in hypertension. This pooled analysis evaluated the antihypertensive effects, organ-protective effects, and safety of esaxerenone in hypertensive patients with CKD by integrating five clinical studies of esaxerenone. Patients were divided based on type 2 diabetes mellitus (T2DM) status (with or without T2DM) and creatinine-based estimated glomerular filtration rate (eGFRcreat) (30 to <60 and ≥60 mL/min/1.73 m2). Significant changes in morning home BP from baseline at Week 12 were observed in the overall population (mean change −12.8/ − 5.4 mmHg), T2DM subgroups ( − 12.2/ − 4.5 and −14.5/ − 7.8 mmHg), and eGFRcreat subgroups ( − 12.5/ − 4.7 and −14.0/ − 6.9 mmHg) (all P < 0.001). Bedtime home and office BP showed similar tendencies. Urine albumin-to-creatinine ratio significantly improved from baseline at Week 12 in the overall population (mean change: −55.2%), T2DM subgroups ( − 56.5% and −52.0%), and eGFRcreat subgroups ( − 54.6% and −55.4%) (all P < 0.001). N-terminal pro-B-type natriuretic peptide levels significantly decreased in the overall population (percent change: −14.1%) and subgroup without T2DM ( − 25.3%). The incidence of serum potassium ≥5.5 mEq/L was lower in the subgroup with T2DM vs without T2DM (3.1% and 11.3%), potentially related to the use of sodium–glucose cotransporter 2 inhibitors. These findings highlight the sustained BP-lowering effect of esaxerenone throughout the day in hypertensive patients with CKD, irrespective of T2DM status, and its significant reduction in albuminuria. The data support the safety and efficacy of esaxerenone in this patient population, underscoring its potential as a valuable therapeutic option.

This study showed that esaxerenone significantly lowered morning home, bedtime home, and office BP and UACR in hypertensive patients with CKD, regardless of T2DM status and kidney function (eGFR), and without any novel safety concerns. These highlight the efficacy, organ-protective effects, and safety of esaxerenone in hypertensive patients with CKD.
Similar content being viewed by others
Introduction
Hypertension is a strong risk factor for the development and progression of chronic kidney disease (CKD) [1,2,3,4]. CKD itself is a major risk factor for cardiovascular morbidity and mortality, particularly in patients with hypertension [5]. The effective management of blood pressure (BP) and reduction of proteinuria are critical in slowing the progression of CKD and reducing associated cardiovascular risks.
In Japan, the 2019 Japanese Society of Hypertension Guidelines for the Management of Hypertension and the 2023 Japanese Society of Nephrology (JSN) Evidence-based Clinical Practice Guidelines for CKD recommend the use of angiotensin receptor blockers (ARBs)/angiotensin converting enzyme inhibitors (ACEis) as first-line treatment for hypertension in patients with CKD and proteinuria [6, 7]. Despite the availability of these guidelines and various antihypertensive agents, achieving optimal BP control remains a challenge for many patients [8]. A recent study demonstrated that patients with hypertension exhibit highly heterogenous treatment effects and cardiovascular prognoses, with not all benefiting from intensive BP treatment [9], which underscores the importance of treating patients with hypertension according to their background characteristics. Real-world data indicate that calcium channel blockers (CCBs) are the most frequently prescribed medications for hypertensive patients with CKD in Japan, followed by ARBs and ACEis [10].
The 2023 JSN Evidence-based Clinical Practice Guidelines emphasize the importance of reducing albuminuria as a therapeutic target, given its strong association with increased mortality in patients with CKD [7]. Renin–angiotensin system (RAS) inhibitors, mineralocorticoid receptor blockers (MRBs), and sodium–glucose cotransporter 2 inhibitors (SGLT2is) are recommended for the management of albuminuria. Recent large-scale studies have shown that SGLT2is provide favorable cardioprotective and renoprotective effects in patients with CKD, regardless of the presence or absence of type 2 diabetes mellitus (T2DM) [11,12,–13], as well as in patients with T2DM alone [14, 15]. Additionally, the MRB finerenone has demonstrated efficacy in suppressing kidney-related adverse events (AEs) in patients with CKD associated with T2DM and has been approved for the treatment of CKD with T2DM, i.e., diabetic nephropathy; however, it is not currently approved for the treatment of hypertension [16,17,18,–19]. This may highlight the need for an MRB with both strong renoprotective and antihypertensive effects.
Esaxerenone, a next-generation non-steroidal MRB, has increased selectivity and potency, a longer half-life, and enhanced bioavailability compared with other MRBs [20, 21]. Esaxerenone has shown favorable BP-lowering effects in hypertensive patients with various characteristics and comorbidities [22,23,24,25,–26], including patients with albuminuria [27,28,29,–30]. In addition to its BP-lowering effect, previous studies have shown the renoprotective effects of esaxerenone, including albuminuria reduction and remission [27,28,29,–30]. However, the efficacy and safety of esaxerenone in hypertensive patients with albuminuria needs to be examined in a wider range of patients, with or without T2DM, under conditions closer to real-world clinical practice.
The aim of this study was to perform a pooled analysis of five clinical studies to evaluate the efficacy, organ-protective effects, and safety of esaxerenone in hypertensive patients with CKD (defined as a urine albumin-to-creatinine ratio [UACR] ≥30 mg/gCr) according to the presence or absence of comorbid T2DM.
Methods
Study design and patients
This study was a pooled subgroup analysis of five clinical studies of esaxerenone: EX-DKD [27], EARLY-NH [24], ESES-LVH [25], ENaK [26], and EAGLE-DH [23]. All studies included were multicenter, prospective, open-label, single-arm trials. Supplementary Table 1 describes the target populations of each study. In all studies, patients received esaxerenone along with basal antihypertensive medications such as ARBs, CCBs, or RAS inhibitors. The details of the eligibility criteria, BP measurements, and biomarker analysis have been previously reported [23,24,25,26,–27].
This subgroup analysis included hypertensive patients with CKD [23,24,25,26,–27], defined as albuminuria (UACR ≥ 30 mg/gCr). Patients were divided into two subgroups based on the presence or absence of comorbid T2DM. Patients were further analyzed in a post hoc subgroup analysis based on creatinine-based estimated glomerular filtration rate (eGFRcreat): 30 to <60 mL/min/1.73 m2 and ≥60 mL/min/1.73 m2. Among the five studies included, two (EAGLE-DH and ESES-LVH) had 24-week treatment periods; however, only data up to 12 weeks were used in this pooled subgroup analysis.
Ethical approval was obtained from the ethical review committee of the Kitamachi Clinic (Tokyo, Japan), and the study was conducted in accordance with the Declaration of Helsinki and local laws and regulations. The requirement for informed consent was waived because of the secondary use of data from previous studies. This pooled analysis study was registered at the University hospital Medical Information Network Clinical Trials Registry (UMIN): UMIN000054922. Each of the five studies were registered in the Japan Registry of Clinical Trials (jRCT) under the following identifiers: jRCTs061190027 (EX-DKD), jRCTs031200364 (EARLY-NH), jRCTs071190043 (ESES-LVH), jRCTs031210273 (ENaK), and jRCTs031200273 (EAGLE-DH).
Study endpoints
The efficacy endpoints included the following: time-course change and change from baseline in morning home, bedtime home, and office systolic BP (SBP)/diastolic BP (DBP) at Week 12; proportion of patients who achieved target BP levels; and change and percent change from baseline in UACR and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) at Week 12. Two criteria were used to define target BP levels in accordance with the Japanese Society of Hypertension 2019 Guidelines (criterion 1: home BP < 135/85 mmHg, office BP < 140/90 mmHg; and criterion 2: home BP < 125/75 mmHg, office BP < 130/80 mmHg for patients aged <75 years, those with CKD [UACR ≥ 30 mg/gCr], or those with diabetes mellitus) [6].
The safety endpoints included the following: treatment-emergent AEs (TEAEs) and adverse drug reactions (ADRs); change from baseline and time-course change in eGFRcreat and serum potassium (K) levels; and proportion of patients with serum K ≥ 5.5 mEq/L within 12 weeks after study drug administration.
The exploratory endpoints were the following: proportion of patients with improved UACR; proportion of patients with a ≥ 30% reduction in UACR from baseline; and proportion of patients with UACR remission. Improved UACR was defined as an improvement in UACR category at Week 12 in patients with baseline UACR A2 or A3 categories. Remission was defined as the transition to the UACR A1 category (UACR < 30 mg/gCr) combined with a ≥ 30% reduction in UACR from baseline. Patients were categorized based on their UACR levels as follows: those with a UACR < 30 mg/gCr were included in the A1 subcohort, those with a UACR of 30 to <300 mg/gCr were included in the A2 subcohort, and those with a UACR of 300 to <1000 mg/gCr were included in the A3 subcohort.
Statistical analysis
No sample size calculations were conducted because this was a pooled analysis of existing trial data. The full analysis set (FAS) of each study was used to evaluate the efficacy endpoints, the per-protocol set (PPS) was used for the sensitivity analysis, and the safety analysis set of each study was used to evaluate the safety endpoints. The definitions for each analysis set have been previously reported [23,24,25,26,–27].
For the difference in BP measurements between baseline and Week 12, point estimates and 95% confidence intervals (CIs) were calculated, and comparisons were made using paired t-tests. The change and percent change from baseline in UACR and NT-proBNP were evaluated using similar significance tests. For the proportion of patients who achieved target BP levels, 95% CIs were calculated using the Clopper–Pearson method. Missing 12-week data were not imputed in this study. TEAEs and ADRs were coded by System Organ Class and Preferred Term according to the Medical Dictionary for Regulatory Activities, version 27.0.
Statistical significance was set at 5% (two-sided). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The total numbers of patients in the safety analysis set and FAS of the five esaxerenone studies were 493 and 479, respectively. Among them, 180 (with T2DM, 127; without T2DM, 53) hypertensive patients with CKD were included in the safety analysis set; 175 (with T2DM, 125; without T2DM, 50) were included in the FAS; and 145 (with T2DM, 102; without T2DM, 43) were included in the PPS.
The background characteristics of patients in the FAS are summarized in Table 1. The proportion of male patients was higher in the subgroup with T2DM vs the subgroup without T2DM (64.8% vs 48.0%). The proportion of patients aged ≥65 years was higher in the subgroup with T2DM vs the subgroup without T2DM (70.4% vs 56.0%). The proportion of patients with body mass index ≥25 kg/m2 was also higher in the subgroup with T2DM vs the subgroup without T2DM (64.0% vs 46.0%). Mean morning home SBP/DBP was 139.3/80.1 and 143.1/87.5 mmHg in the subgroups with and without T2DM, respectively. The mean ± standard deviation (SD) eGFRcreat was lower in the subgroup with T2DM vs the subgroup without T2DM (57.3 ± 16.4 vs 69.3 ± 20.6 mL/min/1.73 m2). Mean ± SD serum K levels were similar in both subgroups (4.2 ± 0.4 and 4.1 ± 0.5 mEq/L, respectively). The final dose of esaxerenone was 1.25 mg in 34.4% and 16.0%; 2.5 mg in 41.6% and 52.0%; and 5 mg in 24.0% and 32.0% of patients in the subgroups with and without T2DM, respectively. The distribution of patients using basal antihypertensive drugs in the overall population was as follows: 34.3% for RAS inhibitors, 26.3% for CCBs, and 39.4% for both drug classes. Similar results were obtained in the PPS (Supplementary Table 2).
Antihypertensive effects
Figure 1 and Supplementary Table 3 show the changes in morning home BP, bedtime home BP, and office BP from baseline at Week 12 in the overall population and in the T2DM and eGFRcreat subgroups. In the overall population, a statistically significant change in morning home SBP/DBP from baseline at Week 12 was observed (mean change: −12.8/ − 5.4 mmHg, P < 0.001; Fig. 1a). This BP reduction was consistent in both subgroups with and without T2DM (mean change: −12.2/ − 4.5 and −14.5/ − 7.8 mmHg, respectively, both P < 0.001; Fig. 1b). It was also consistent in both subgroups with eGFRcreat 30 to <60 and ≥60 mL/min/1.73 m2 (mean change: −12.5/ − 4.7 and −14.0/ − 6.9 mmHg, respectively, both P < 0.001; Fig. 1c). The bedtime home and office SBP/DBP showed similar tendencies (Fig. 1d–i). The changes in morning home BP, bedtime home BP, and office BP from baseline to Week 12 in the PPS are shown in Supplementary Table 4.
Changes in morning home BP (a–c), bedtime home BP (d–f) and office BP (g–i) in the overall population, T2DM subgroups, and eGFRcreat subgroups (full analysis set). Mean, error bar (95% CI). ***P < 0.001 vs baseline. BP blood pressure, CI confidence interval, DBP diastolic BP, eGFRcreat creatinine-based estimated glomerular filtration rate, SBP systolic BP, T2DM type 2 diabetes mellitus
The proportions of patients who achieved target BP levels are shown in Fig. 2 and Supplementary Table 5. The proportions of patients who achieved target home SBP/DBP < 135/85 mmHg and office SBP/DBP < 140/90 mmHg in the overall population were as follows: 66.9% for morning home BP, 73.5% for bedtime home BP, and 69.2% for office BP. The proportions of patients who achieved target home SBP/DBP < 125/75 mmHg and office SBP/DBP < 130/80 mmHg in the overall population were as follows: 19.9% for morning home BP, 41.7% for bedtime home BP, and 39.1% for office BP. The percentage of patients who achieved target BP levels was numerically higher in the subgroup with T2DM than in the subgroup without T2DM (no statistical tests were performed). Achievement rates of target BP levels by eGFRcreat subgroups were not analyzed.
Effects on UACR
The UACR showed a statistically significant improvement from baseline at Week 12 in the overall population (mean change: −55.2%, P < 0.001); the subgroups with and without T2DM ( − 56.5% and −52.0%), respectively, both P < 0.001; and the subgroups with eGFRcreat 30 to <60 and ≥60 mL/min/1.73 m2 (−54.6% and −55.4%, respectively, both P < 0.001) (Fig. 3a and Supplementary Table 6).
Percentage change in UACR from baseline to Week 12 (a) and improvement rates of UACR (b) in the overall population, T2DM subgroups, and eGFRcreat subgroups (full analysis set). a Mean, error bar (95% CI); ***P < 0.001 vs baseline. b A2/A3. aRemission was defined as the transition to UACR A1 combined with a ≥ 30% reduction in UACR from baseline. A1 UACR < 30 mg/gCr, A2 UACR 30 to <300 mg/gCr, A3 UACR 300–1000 mg/gCr, CI confidence interval, eGFRcreat creatinine-based estimated glomerular filtration rate, T2DM type 2 diabetes mellitus, UACR urine albumin-to-creatinine ratio
The proportion of patients with UACR improvement, ≥30% reduction in UACR, and UACR remission are shown in Fig. 3b and Supplementary Table 7. UACR improved in 44.9% of all patients, 42.7% of patients with T2DM, 50.0% of patients without T2DM, 39.4% of patients with eGFRcreat 30 to <60 mL/min/1.73 m2, and 54.5% of patients with eGFRcreat ≥ 60 mL/min/1.73 m2. UACR improvement and UACR remission were similar between T2DM subgroups and between eGFRcreat subgroups. The proportion of patients with ≥30% reduction in UACR was 71.2% of all patients, 70.9% of patients with T2DM, 71.7% of patients without T2DM, 69.1% of patients with eGFRcreat 30 to <60 mL/min/1.73 m2, and 76.4% of patients with eGFRcreat ≥ 60 mL/min/1.73 m2. In the A2 subcohort, remission of albuminuria was achieved in 41.2% of all patients, 38.2% of patients with T2DM, 47.4% of patients without T2DM, 33.9% of patients with eGFRcreat 30 to <60 mL/min/1.73 m2, and 51.0% of patients with eGFRcreat ≥ 60 mL/min/1.73 m2 (Supplementary Table 7). In the A3 subcohort, remission of albuminuria was achieved in 7.1% of all patients, 5.9% of patients with T2DM, 12.5% of patients without T2DM, 2.9% of patients with eGFRcreat 30 to <60 mL/min/1.73 m2, and 16.7% of patients with eGFRcreat ≥ 60 mL/min/1.73 m2 (Supplementary Table 7). In the A2 + A3 subcohort, remission of albuminuria was achieved in 32.1% of all patients, 28.2% of patients with T2DM, 41.3% of patients without T2DM, 22.3% of patients with eGFRcreat 30 to <60 mL/min/1.73 m2, and 47.3% of patients with eGFRcreat ≥ 60 mL/min/1.73 m2 (Fig. 3b; Supplementary Table 7). Similar tendencies were observed in the PPS (Supplementary Table 8).
NT-proBNP levels significantly decreased from baseline to Week 12 in the overall population (percent change: −14.1%, P < 0.001) (Supplementary Table 6). The percent changes in NT-proBNP levels from baseline to Week 12 were −8.7% in the subgroup with T2DM and −25.3% in the subgroup without T2DM, but the change only reached statistical significance in the group without T2DM (P < 0.001). NT-proBNP levels by eGFRcreat subgroups were not analyzed.
Safety
The safety results are summarized in Table 2. In the overall population, the incidence of TEAEs was 28.9%; serious TEAEs, 2.2%; ADRs, 8.9%; and serious ADRs, 0%. These results were similar between T2DM subgroups. TEAEs and ADRs by eGFRcreat subgroups were not analyzed. In the overall population, the most frequent ADRs were hyperkalemia and blood potassium increased, each in 2.8% of patients. Among the five clinical studies of esaxerenone included in this pooled analysis, no cases of acute kidney injury were reported as TEAEs.
After starting treatment with esaxerenone, serum K levels increased up to Week 2; thereafter, levels remained stable up to Week 12 (Fig. 4a and Supplementary Table 9). Similar trends were observed in both T2DM subgroups and eGFRcreat subgroups (Fig. 4b, c). The incidence of serum K ≥ 5.5 mEq/L was 5.6% (10/180 patients) in the overall population (Supplementary Table 10). The incidence of serum K ≥ 5.5 mEq/L was numerically lower in the subgroup with T2DM (3.1% [4/127 patients]) than in the subgroup without T2DM (11.3% [6/53 patients]); however, no statistical tests were performed. The incidence of serum K ≥ 5.5 mEq/L was slightly higher in patients with eGFRcreat 30 to <60 mL/min/1.73 m2 (5.5% [6/110 patients]) than in patients with eGFRcreat ≥ 60 mL/min/1.73 m2 (3.2% [2/62 patients]) (Supplementary Table 10).
After starting esaxerenone treatment, the eGFRcreat decreased up to Week 2 and remained stable thereafter up to Week 12 in the overall population, in the T2DM subgroups, and in the eGFRcreat subgroups (Fig. 4d–f). The subgroup with T2DM had a lower baseline eGFRcreat level than the subgroup without T2DM (Fig. 4e and Supplementary Table 9).
Discussion
This pooled analysis of five clinical studies showed that esaxerenone significantly lowered morning home BP, bedtime home BP, and office BP in hypertensive patients with albuminuria, regardless of the presence or absence of T2DM and kidney function based on eGFRcreat subgroup category (30 to <60 and ≥60 mL/min/1.73 m2). Additionally, esaxerenone treatment reduced albuminuria, as evidenced by the significant reduction in UACR in both patient groups, supporting its renoprotective effects. NT-proBNP levels significantly decreased in the overall population and in patients without T2DM, but not in those with T2DM. The overall safety profile of esaxerenone was similar in patients with and without T2DM and in the eGFRcreat subgroups. The incidence of serum K ≥ 5.5 mEq/L was numerically higher in patients without T2DM than in those with T2DM, which may be related to the differences in the use of SGLT2is between the two groups.
Previous studies have shown that esaxerenone is effective in reducing BP and albuminuria in hypertensive patients with CKD (albuminuria) [27,28,29,–30]. Our findings are consistent with these results, further confirming the broad antihypertensive efficacy of esaxerenone. Furthermore, our study builds upon these findings by demonstrating that the renoprotective effects of esaxerenone persist regardless of T2DM status.
In this study, esaxerenone was found to significantly lower morning home BP, bedtime home BP, and office BP in hypertensive patients with CKD. The ultimate goal of antihypertensive therapy is to achieve optimal BP control, thus minimizing target organ damage and cardiovascular events [31]. Therefore, achieving consistent 24-hour BP control is important to reduce cardiovascular events [32,33,–34]. However, the HI-JAMP study reported that 45%–55% of participants had uncontrolled nocturnal and/or morning hypertension during treatment with three or more antihypertensive drugs [8]. In the present study, esaxerenone lowered BP at all time points (during the morning, office hours, and at bedtime), regardless of the presence or absence of T2DM or kidney function based on eGFRcreat. This suggests that the BP-lowering effects of esaxerenone are sustained throughout the day across diverse patient profiles. The difference in BP change between subgroups may be influenced by the fact that patients with T2DM and eGFRcreat 30 to <60 mL/min/1.73 m2 were started on esaxerenone at 1.25 mg, with many still using lower doses at 12 weeks.
Albuminuria was improved and a decrease in UACR was observed. This suggests that esaxerenone has a renoprotective effect, with 55.2% reduction in UACR, independent of T2DM status or kidney function by eGFRcreat. This reduction of UACR in patients with albuminuria was comparable with the results of previous Phase 3 studies of esaxerenone in T2DM patients with albuminuria, reporting reductions of 32.4% [28], 43.8% [29], and 34.4% at 12 weeks [30]. This reduction in UACR was also comparable with that of previous studies of finerenone, which showed a 31% reduction at 4 months in the FIDELIO-DKD trial [19] and a 32% greater reduction with finerenone versus placebo at 4 months in the FIGARO-DKD trial [18]. Those previous studies showed that finerenone significantly reduced the risk of CKD progression and cardiovascular events compared with placebo [18, 19]. To date, although the long-term effects of esaxerenone on kidney and cardiovascular outcomes have not been examined, its renoprotective effect, based on improvements in UACR and UACR classification, is considered clinically meaningful. Esaxerenone is a selective MRB that works by inhibiting the effects of aldosterone, a hormone that increases BP and promotes kidney damage. The reduction in UACR observed in our study suggests that esaxerenone effectively mitigates aldosterone-induced kidney damage.
NT-proBNP levels decreased significantly in the overall population ( − 14.1%, P < 0.001), but the decrease was not statistically significant in patients with T2DM, possibly because of differences in baseline NT-proBNP levels between patients with and without T2DM (123.2 ± 184.0 and 157.5 ± 232.0 pg/mL, respectively); NT-proBNP levels at 12 weeks were similar between the two T2DM subgroups (142.3 ± 300.3 and 134.4 ± 211.4 pg/mL, respectively). It should be noted that in this study, only 47 (26.9%) patients had NT-proBNP levels ≥125 pg/mL at baseline, and the majority were within the normal range. The ESES-LVH study [25], one of the five clinical studies used in this pooled analysis, showed the cardioprotective effects of esaxerenone based on the reduction in NT-proBNP and left ventricular mass index (LVMI) in hypertensive patients with left ventricular hypertrophy. Additionally, recent findings suggest that increased plasma renin activity induced by MRBs, without concurrent RAS inhibition, may be associated with reduced muscle mass in patients with heart failure [35], although muscle wasting and LVMI reduction are distinct phenomena. This may warrant caution when treatment with MRBs is prescribed for patients with heart failure not receiving RAS inhibitors. Thus, further studies are needed to confirm the cardioprotective effects of esaxerenone.
The percentage of patients with serum K ≥ 5.5 mEq/L was higher in patients without T2DM than in those with T2DM (11.3% vs 3.1%). This difference may be due to differences in the frequency of SGLT2i use between patients with and without T2DM, as well as differences in the esaxerenone dose at last administration. Several studies have reported that SGLT2is decrease the risk of hyperkalemia when administered in combination with an MRB including esaxerenone in patients with T2DM [23, 36,37,38,39]. Patients with an eGFRcreat 30 to <60 mL/min/1.73 m2 had a slightly higher incidence of serum K ≥ 5.5 mEq/L than those with eGFRcreat ≥ 60 mL/min/1.73 m2, despite the lower final dose of esaxerenone. Because reduced kidney function is a known risk factor for hyperkalemia during MRB use, esaxerenone should be administered with greater caution in patients with reduced eGFRcreat compared to those with normal kidney function [22].
The results of this study have important implications for the clinical management of hypertensive patients with albuminuria. The ≥10 mmHg reduction in SBP and 50% improvement in UACR are clinically significant findings and may lead to changes in classification (e.g., from macroalbuminuria to microalbuminuria or from microalbuminuria to normoalbuminuria). Although reduction in UACR has not been consistently linked with hard outcomes in some clinical trials, such as ALTITUDE or VA NEPHRON-D [40, 41], reducing proteinuria remains clinically important. While UACR reduction may serve as a potential surrogate marker for renoprotection, definitive evidence linking it to clinical outcomes would require event-driven trials. The present study findings suggest that esaxerenone may offer a valuable treatment option for hypertensive patients with albuminuria, potentially improving outcomes and reducing the risk of cardiovascular and kidney complications. Additionally, the data on eGFRcreat and serum K underscore the safety of this protocol. Nevertheless, the cardioprotective effects of esaxerenone need to be verified in future studies.
Limitations
This study has some limitations that should be considered in the interpretation of its findings. First, data were reported up to 12 weeks only, which may not capture long-term effects. Second, the study population was limited to Japanese patients, and the results may not be generalizable to other populations. Third, this was an analysis of secondary data, which may have introduced bias and influenced the results. Fourth, all findings presented in this study are based on data from single-arm studies, and the lack of comparator groups is a limitation. Fifth, the decision to increase the esaxerenone dose was made by the physician based on the patient’s condition, which may have resulted in a lower rate of achieving antihypertensive control. Sixth, the possibility of type I error should also be considered because corrections for multiplicity were not applied. Seventh, although the study protocol set exclusion criteria to eliminate patients with secondary hypertension, a definitive diagnosis of primary aldosteronism was not performed, so the possibility that patients with primary aldosteronism were included cannot be ruled out. Eighth, only baseline values of plasma aldosterone and renin activity were assessed, and changes following the start of esaxerenone administration were not evaluated. Finally, no statistical tests were performed to compare differences between the patient subgroups.
Conclusion
The results of this pooled subanalysis demonstrated that esaxerenone significantly lowered morning home, bedtime home, and office BP in hypertensive patients with CKD, regardless of the presence or absence of T2DM. Additionally, esaxerenone treatment improved albuminuria, as evidenced by the significant reduction in UACR in both patient subgroups (by T2DM status and kidney function), supporting its renoprotective effects. NT-proBNP levels significantly decreased in the overall population and in patients without T2DM, but not in those with T2DM, suggesting that the cardioprotective effects of esaxerenone may be limited in patients with T2DM. The overall safety profile of esaxerenone was similar in patients with and without T2DM. The incidence of serum K ≥ 5.5 mEq/L was numerically higher in patients without T2DM than in those with T2DM, which may be related to differences in the use of SGLT2is between the two groups. These findings highlight the efficacy, organ-protective effects, and safety of esaxerenone in hypertensive patients with CKD and warrant further investigation in future studies.
Data availability
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. Data disclosure can be requested for 36 months from article publication.
References
Lohr JW, Golzy M, Carter RL, Arora P. Elevated systolic blood pressure is associated with increased incidence of chronic kidney disease but not mortality in elderly veterans. J Am Soc Hypertens. 2015;9:29–37.
Inker LA, Tighiouart H, Aspelund T, Gudnason V, Harris T, Indridason OS, et al. Lifetime risk of stage 3-5 CKD in a community-based sample in Iceland. Clin J Am Soc Nephrol. 2015;10:1575–84.
Hirayama A, Konta T, Kamei K, Suzuki K, Ichikawa K, Fujimoto S, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28:1150–6.
Kaneyama A, Hirata A, Hirata T, Imai Y, Kuwabara K, Funamoto M, et al. Impact of hypertension and diabetes on the onset of chronic kidney disease in a general Japanese population. Hypertens Res. 2023;46:311–20.
Jin Q, Mei J, Wong YC, Lam CLK, Wan EYF. Associations and attributable burden between risk factors and all-cause and cause-specific mortality at different ages in patients with hypertension. Hypertens Res. 2024;47:2053–63.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Japanese Society of Nephrology. Essential points from evidence-based clinical practice guideline for chronic kidney disease 2023. Clin Exp Nephrol. 2024;28:473–95.
Kario K, Tomitani N, Nishizawa M, Harada N, Kanegae H, Hoshide S. Concept, study design, and baseline blood pressure control status of the nationwide prospective HI-JAMP study using multisensor ABPM. Hypertens Res. 2023;46:357–67.
Zhan R, Zhang J, Chen X, Liu T, He Y, Zhang S, et al. Targeting the efficacy of intensive blood pressure treatment in hypertensive patients - an exploratory analysis of SPRINT. Circ J. 2023;87:1212–8.
Tada K, Nakano Y, Takahashi K, Hiyamuta H, Watanabe M, Ito K, et al. Current use of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors for hypertension in patients with chronic kidney disease with proteinuria: a cross-sectional study based on real-world data. Hypertens Res. 2025;48:244–55.
Reyes-Farias CI, Reategui-Diaz M, Romani-Romani F, Prokop L. The effect of sodium-glucose cotransporter 2 inhibitors in patients with chronic kidney disease with or without type 2 diabetes mellitus on cardiovascular and renal outcomes: A systematic review and meta-analysis. PLoS One. 2023;18:e0295059.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N. Engl J Med. 2020;383:1436–46.
Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diab Endocrinol. 2021;9:22–31.
Dekkers CCJ, Gansevoort RT. Sodium-glucose cotransporter 2 inhibitors: extending the indication to non-diabetic kidney disease? Nephrol Dial Transpl. 2020;35:i33–i42.
Chilton RJ. Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diab Obes Metab. 2020;22:16–29.
Singh AK, Singh A, Singh R, Misra A. Finerenone in diabetic kidney disease: a systematic review and critical appraisal. Diab Metab Syndr. 2022;16:102638.
Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143:540–52.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl J Med. 2021;385:2252–63.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl J Med. 2020;383:2219–29.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharm. 2015;761:226–34.
Janković SM, Janković SV. Clinical pharmacokinetics and pharmacodynamics of esaxerenone, a novel mineralocorticoid receptor antagonist: a review. Eur J Drug Metab Pharmacokinet. 2022;47:291–308.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85.
Motoki H, Inobe Y, Fukui T, Iwasaki A, Hiramitsu S, Koyama S, et al. Efficacy and safety of esaxerenone in hypertensive patients with diabetes mellitus undergoing treatment with sodium-glucose cotransporter 2 inhibitors (EAGLE-DH). Adv Ther. 2023;40:5055–75.
Kario K, Nishizawa M, Kato M, Ishii H, Uchiyama K, Nagai M, et al. Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study. Hypertens Res. 2023;46:1782–94.
Yamamoto E, Usuku H, Sueta D, Suzuki S, Nakamura T, Matsui K, et al. Efficacy and safety of esaxerenone in hypertensive patients with left ventricular hypertrophy (ESES-LVH) study: a multicenter, open-label, prospective, interventional study. Adv Ther. 2024;41:1284–303.
Katsuya T, Inobe Y, Uchiyama K, Nishikawa T, Hirano K, Kato M, et al. Exploratory study on the relationship between urinary sodium/potassium ratio, salt intake, and the antihypertensive effect of esaxerenone: the ENaK Study. Hypertens Res. 2024;47:835–48.
Uchida HA, Nakajima H, Hashimoto M, Nakamura A, Nunoue T, Murakami K, et al. Efficacy and safety of esaxerenone in hypertensive patients with diabetic kidney disease: a multicenter, open-label, prospective study. Adv Ther. 2022;39:5158–75.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): Phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15:1715–27.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021;25:1070–8.
Kario K. What are the ideal metrics for assessing the quality of long-term stabilized “perfect” 24-h BP control after renal denervation? Hypertens Res. 2024;47:2644–51.
Lauder L, Mahfoud F, Azizi M, Bhatt DL, Ewen S, Kario K, et al. Hypertension management in patients with cardiovascular comorbidities. Eur Heart J. 2023;44:2066–77.
Kario K, Shin J, Chen CH, Buranakitjaroen P, Chia YC, Divinagracia R, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: The HOPE Asia Network. J Clin Hypertens (Greenwich). 2019;21:1250–83.
Tomitani N, Hoshide S, Kario K. HI-JAMP study investigators. Diagnostic agreement of masked uncontrolled hypertension detected by ambulatory blood pressure and home blood pressure measured by an all-in-one BP monitoring device: The HI–JAMP study. Hypertens Res. 2023;46:157–64.
Numazawa R, Katano S, Yano T, Nagaoka R, Ohori K, Kouzu H, et al. Independent link between use of mineralocorticoid receptor antagonists and muscle wasting in heart failure patients not receiving renin-angiotensin system inhibitors. Circ J. 2023;88:10–9.
Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036–9.
Kristensen SL, Docherty KF, Jhund PS, Bengtsson O, Demets DL, Inzucchi SE, et al. Dapagliflozin reduces the risk of hyperkalaemia in patients with heart failure and reduced ejection fraction: a secondary analysis DAPA-HF. Eur Heart J. 2020;41:ehaa946.0939.
Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep. 2021;7:36–45.
Shikata K, Ito S, Kashihara N, Nangaku M, Wada T, Okuda Y, et al. Reduction in the magnitude of serum potassium elevation in combination therapy with esaxerenone (CS-3150) and sodium-glucose cotransporter 2 inhibitor in patients with diabetic kidney disease: Subanalysis of two phase III studies. J Diab Investig. 2022;13:1190–202.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13.
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl J Med. 2013;369:1892–903.
Acknowledgements
We thank Michelle Belanger, MD, of Edanz (www.edanz.com) for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd., in accordance with Good Publication Practice 2022 guidelines (https://www.ismpp.org/gpp-2022).
Funding
This study was supported by Daiichi Sankyo Co., Ltd., which was involved in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in the data management or statistical analysis. Data management and statistical analysis were performed by Satt Co., Ltd.
Author information
Authors and Affiliations
Contributions
All authors fulfilled the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
HAU has no conflicts of interest to declare. JW received honoraria for speakers’ bureaus from AstraZeneca K.K. and Daiichi Sankyo Co., Ltd. HM has no conflicts of interest to declare. KK received research funding from Novo Nordisk Pharma Ltd., and Kowa Co., Ltd.; honoraria from MSD K.K., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., AstraZeneca K.K., Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin, Ltd., and Pfizer Japan Inc.; scholarships or donations from Otsuka Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Taisho Pharmaceutical Co., Ltd., and Fukuda Denshi Co., Ltd.; and departmental endowments from Abbott Medical Japan L.L.C., Medtronic Japan Co., Ltd., Biotronik Japan, Inc., Boston Scientific Japan K.K., Japan Lifeline Co., Ltd., Terumo Corporation, Nipro Corporation, and Cordis Japan G.K. KK received support for medical writing, article processing charges, research funding, and advisory fees from Daiichi Sankyo Co., Ltd.; grants from Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., MSD K.K., Sumitomo Pharma Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd.; consulting fees from Sanwa Chemical Co., Ltd.; honoraria from Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., and Viatris Pharmaceuticals Japan G.K.; and participated in advisory boards for Novartis Pharma K.K. and Daiichi Sankyo Co., Ltd. TK received honoraria from Daiichi Sankyo Co., Ltd., CureApp, Inc., and Novartis Pharma K.K. TS had an employment/leadership position/advisory role with Sekisui Medical Co., Ltd., and EP Mediate Co., Ltd.; and received honoraria from Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., AEON Retail Co., Ltd., and AEON Happicom Co., Ltd. KT received honoraria from Abbott Medical Co., Ltd., Amgen K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., and Terumo Corporation; research funding from Mochida Pharmaceutical Co., Ltd. and EA Pharma Co., Ltd.; scholarships or donations from Abbott Medical Japan L.L.C., ITI Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Boston Scientific Japan K.K.; and departmental endowments from Abbott Japan L.L.C., Boston Scientific Japan K.K., Fides-one, Inc., GM Medical Co., Ltd., ITI Co., Ltd., Kaneka Medix Coporation, Nipro Corporation, Terumo Coporation, Philips Japan, Ltd., Getinge Group Japan K.K., Orbusneich Medical K.K., Abbott Medical Japan L.L.C., Biotronik Japan, Inc., Boston Scientific Japan K.K., Fukuda Denshi Co., Ltd., Japan Lifeline Co., Ltd., Medtronic Japan Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. SS, TS, and TT are employees of Daiichi Sankyo Co., Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior publication: Some results of this study have been submitted as a congress abstract to the 68th Annual Meeting of the Japanese Society of Nephrology (June 20–22, 2025; Yokohama, Japan), which is supported by Daiichi Sankyo Co., Ltd.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uchida, H.A., Wada, J., Motoki, H. et al. Efficacy and safety of esaxerenone in hypertensive patients with chronic kidney disease, with or without type 2 diabetes mellitus: a pooled analysis of five clinical studies. Hypertens Res 48, 2413–2426 (2025). https://doi.org/10.1038/s41440-025-02259-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02259-z
Keywords
This article is cited by
-
Aldosterone-mediated hypertension and non-steroidal mineralocorticoid receptor antagonists
Hypertension Research (2025)
-
Expanding the therapeutic frontier: esaxerenone in hypertensive patients with CKD and albuminuria
Hypertension Research (2025)