Abstract
Intracerebral renin-angiotensin system (RAS) activation in the forebrain nuclei, such as the subfornical organ (SFO) is among the important factors that increase sodium appetite in rats. Although an increased sodium appetite in congestive heart failure and spontaneously hypertensive rats was reported previously, few reports have shown an increased sodium appetite in chronic kidney disease (CKD) model rats, for which salt restriction is important. Here, we aimed to verify whether CKD model rats show increased sodium appetite and if therapeutic intervention reducing sodium appetite by suppressing the intracerebral RAS is possible. In this study, 5/6 nephrectomized (5/6Nx) and control rats were fed a high-salt (4% NaCl) or low-salt (0.04% NaCl) diet. Angiotensin II (AngII) levels in kidney and the SFO, renal injury, and sodium appetite were evaluated after 2 weeks of salt loading. The 5/6Nx high-salt diet (Nx-HS) group was further divided into subgroups receiving continuous intracerebroventricular (ICV) administration of the angiotensin II type 1 receptor (AT1R) antagonist ZD 7155 or saline. Compared with the control rats, the 5/6Nx rats exhibited significantly increased levels of AngII in kidney and the SFO, accompanied by elevated blood pressure and worsened renal injury, especially in the Nx-HS group. In the Nx-HS group, an increased sodium appetite was observed that was attenuated by the ICV administration of ZD 7155 but not by saline. In conclusion, a high-salt diet enhanced the sodium appetite of 5/6Nx rats via increased levels of AngII in the SFO, which was attenuated by continuous ICV administration of an AT1R antagonist.

Similar content being viewed by others
Introduction
The salt restriction is the cornerstone of treatment in patients with chronic kidney disease (CKD), heart failure, and hypertension; however, it is known that compliance with a prescribed reduced-sodium diet in these patients is extremely low. Sodium appetite, a motivational state that involves seeking out and ingesting salty substances, is operationally identified by measuring hypertonic sodium solution consumption under specified experimental conditions [1]. Sodium appetite was reportedly increased in a congestive heart failure rat model [2], spontaneously hypertensive rat model [3], and renovascular hypertension rat model [4]. Thus, we hypothesize that an increased sodium appetite in patients with CKD makes it difficult for them to decrease their salt intake.
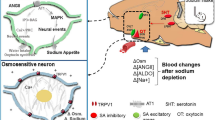
The renin-angiotensin system (RAS) of the circulation and in the kidney plays a critical role in maintaining sodium homeostasis. Although the effect of RAS on sodium excretion has been well studied in a CKD model, its effects on sodium intake are unknown. Multiple factors regulate sodium appetite and much remains unclear; however, angiotensin II (AngII) stimulation in the subfornical organ (SFO) is thought to contribute to sodium appetite [5, 6]. The SFO is among the forebrain circumventricular organs, a collection of structures that reside outside the blood-brain barrier, are directly exposed to the peripheral milieu, and express a high density of AngII type I receptor (AT1R) [7]. The SFO projects to multiple cerebral nuclei and has various suspected functions. The SFO–ventral bed nucleus of the stria terminalis (vBNST) pathway was recently shown to drive sodium appetite [8] via circulating AngII activation [9, 10]. Previous studies used experimental models that enhanced circulating RAS, such as diuretic agent (e.g. furosemide) administration, dietary sodium deprivation, and colloid-induced hypovolemia, or direct AngII administration, to induce sodium appetite [11,12,13,14]. Moreover, several studies demonstrated that electrolytic lesions of the SFO drastically attenuated salt appetite in sodium-depleted rats [15, 16].
Cao et al. reported that 5/6 nephrectomized rats that received a high-salt (4% NaCl) diet had an activated intrarenal and intracerebral RAS through the reno-cerebral reflex, despite the lack of circulating RAS [17]. Although sodium appetite was not evaluated in this model, the intracerebroventricular (ICV) administration of losartan was confirmed to attenuate intracerebral RAS activity in forebrain nuclei such as the SFO. Here, we aimed to verify whether CKD model rats would show increased sodium appetite via activated intracerebral RAS and determine the feasibility of a therapeutic intervention that suppresses intracerebral RAS.
Methods
Experimental animals
All animal procedures were conducted with the approval of the Animal Committee of the Hamamatsu University School of Medicine. Six-week-old male Sprague-Dawley rats weighing 160–180 g were purchased from SLC (Hamamatsu, Japan) and housed in individual cages under standard laboratory conditions (temperature, 24 ± 2°C; humidity, 55 ± 5%; light/dark cycle, 12/12 h).
Treatments
Protocol 1
The 5/6 nephrectomy (5/6Nx) or sham operation was performed in 6-week-old rats as previously described [18]. Postoperatively, all rats received a normal-salt (0.4% NaCl) diet for 2 weeks and were then randomly assigned to the following four groups (n = 10 each): group C, sham operation and a low-salt (0.04% NaCl) (C-LS) or high-salt (4% NaCl) (C-HS) diet for 2 weeks; group Nx, 5/6Nx and a low-salt (0.04% NaCl) (Nx-LS) or high-salt (4% NaCl) (Nx-HS) diet for 2 weeks. After that, in all groups, the diet was changed to an LS diet, and after a 3-day washout period, sodium appetite was evaluated by a 4-day two-bottle test. Finally, blood samples were taken of all rats, which were then sacrificed by perfusion fixation with 10% formalin, and brain and kidney samples were collected.
Protocol 2
After 5/6Nx was performed at 6 weeks of age, all rats were randomly assigned to the following two groups (n = 10 per group): group Nx-HS-C, the 5/6Nx rats receiving the ICV administration of vehicle (normal saline solution) using an Alzet osmotic pump (Durect Corp.) [17] for 4 weeks and an HS (4% NaCl) diet for 2 weeks, or group Nx-HS-Z, the 5/6Nx rats receiving ICV administration of ZD 7155 (AT1 receptor antagonist; Sigma Aldrich) using an Alzet osmotic pump for 4 weeks and an HS (4% NaCl) diet for 2 weeks. The evaluation of sodium appetite and sacrifice was performed as in Protocol 1.
The schema of the experimental protocols is shown in Supplementary Fig. 1.
Brain surgery
Cannulation of the rat lateral ventricle was performed as described previously [19]. Briefly, the rats were anesthetized with an intraperitoneal injection of ketamine (Daiichisankyo; 90 mg/kg body weight [BW]) combined with xylazine (Bayer; 10 mg/kg BW) and placed in a stereotaxic apparatus, and the skull was leveled between the bregma and lambda. An Alzet Brain Infusion Kit 2 (a stainless steel 28-gauge canula) was implanted into the right lateral ventricle (LV) using the following coordinates: 0.8 mm caudal to the bregma, 1.6 mm lateral to the bregma, and 4.3 mm below the surface of the skull. The coordinate position was determined with reference to the Paxinos & Watson rat brain atlas. The brain infusion kit was connected to an Alzet osmotic pump (2ML4) via a vinyl catheter tube, and the osmotic pump was implanted subcutaneously. The rats were allowed to recover for 1 week before starting the salt loading.
Brain drug delivery
We dissolved ZD 7155 hydrochloride, the potent AT1R antagonist [20], in 2 mL of saline to adjust the concentration to 0.1 mg/kg BW/day, and filled the solution into the osmotic pump in Nx-HS-Z group. The osmotic pumps in the Nx-HS-C group were filled with saline only.
Two-bottle test
To evaluate sodium appetite, we performed a two-bottle test at the end of the experimental period as previously described [2, 3, 21]. All rats were housed in individual cages and given free access to a low-salt diet and two 200 mL drinking bottles, one of which contained tap water and the other 0.9% saline. To limit the rats’ salt intake to that of the saline only, all rats were fed a low-salt diet during the two-bottle test. Following a 3-day acclimatization period, we conducted a 4-day two-bottle test. The fluid intake of each rat was measured daily at 0:00 pm. All bottles were cleaned and refilled with fresh water and 0.9% saline, and the left-right positions of the water and saline bottles were alternated daily to control for side preferences. An average daily fluid intake was calculated and expressed per 100 g of BW. Sodium appetite was assessed by measuring the intake of saline, and salt preference was defined as the ratio of saline intake to the sum of tap water and saline intake.
Measurement of systolic blood pressure, renal function, and circulating AngII and Ang-(1-7)
Systolic blood pressure (SBP) was measured using a noninvasive tail-cuff method (BP98A; Softron, Tokyo, Japan), before euthanasia. The blood samples were collected before perfusion fixation. Serum creatinine (sCr) concentrations were measured by enzymatic assays (Sanritsu Zelkova Laboratory, Kyoto, Japan). Plasma AngII levels were measured by enzyme-linked immunosorbent assay kits (Elabscience, Houston, TX, USA) as has been done in our previous studies [18, 22,23,24]. We also measured plasma angiotensin (1-7) (Ang-(1-7)) levels in protocol 2 by ELISA kits (Elabscience, Houston, TX, USA).
Evaluation of glomerular and tubulointerstitial lesions
Kidney tissues were fixed in 10% formaldehyde in phosphate-buffered saline and embedded in paraffin. Tissue sections were stained with periodic acid–Schiff and Masson’s trichrome stains for the histopathological evaluation of glomerular and tubulointerstitial lesions. The extent of glomerular sclerosis was assessed semi-quantitatively as described previously [25, 26]. Briefly, at least 50 glomeruli from each kidney were graded on the periodic acid–Schiff-stained sections according to the following criteria: 0, no sclerosis; 1, <25% exhibiting cross-sectional sclerosis; 2, 25–50% exhibiting sclerosis; 3, 50–75% exhibiting sclerosis; and 4, >75% exhibiting cross-sectional sclerosis. The sclerosis index for each rat was calculated as follows: (N1 × 1 + N2 × 2 + N3 × 3 + N4 × 4)/n, where N1, N2, N3, and N4 represent the numbers of glomeruli that exhibited grades 1, 2, 3, and 4, respectively, and n represents the number of glomeruli assessed. Similarly, renal fibrosis extent was quantitated on the Masson’s trichrome–stained sections from each kidney. The percentages of tubulointerstitial fibrosis were evaluated in microscopic fields observed at 100× magnification. Ten microscopic fields were evaluated for each rat using a point-counting method and mean values calculated as described previously [22]. All quantitative analyses were performed in a blinded manner to avoid bias.
Immunohistochemical analysis
AGT and AngII expression in kidney
Kidney samples were sectioned at 3-μm intervals and immunostained for angiotensinogen (AGT) and AngII using a Histofine kit (Nichirei-Bioscience, Tokyo, Japan) as previously described [18, 22, 23, 27]. The primary antibodies were rabbit anti-AGT antibody (IBL Co. Ltd., Takasaki, Japan) and rabbit anti-AngII antibody (Phoenix Pharmaceuticals, Burlingame, CA, USA). The immunoreactivity of AGT and AngII was quantitatively evaluated by a semiautomatic image analysis system using ImageJ software (ver 1.52a; National Institutes of Health, Bethesda, MD, USA) [24]. Twenty random microscopic fields (400×) in the renal cortex were examined for each slide, and the averages of AGT- and AngII-positive areas, excluding glomerular and vascular lesions, were obtained.
AGT and AngII expression in brain
The intracerebral RAS was assessed in the SFO using AGT and AngII expression analyses [17]. The rats were anesthetized and perfused as described above. The whole brains were dissected and cut into thick coronal slices (2 mm) with a rat brain slicer matrix (Zivic Instruments). The slices were post-fixed overnight at 4 °C in 10% formaldehyde, embedded in paraffin, and cut into serial 2-μm-thick sections. The SFO was identified under microscopy, and immunohistochemical staining was performed as described above. The number of AGT- and AngII-positive cells were counted in each slide, and the results are expressed as number of positive cells per 104 μm using ImageJ software.
Statistical analysis
The results are expressed as mean ± standard deviation. Significant intergroup differences in Protocol 1 were determined using analysis of variance, followed by Tukey’s or Games–Howell’s post-hoc analysis as appropriate. A t-test was performed for the groups in Protocol 2. Values of P < 0.05 were considered statistically significant. The statistical analyses were performed using SPSS software version 25 (SPSS, Chicago, IL, USA).
Results
Induction of high SBP, renal damage and increased levels of intrarenal AngII by HS intake in 5/6Nx rats
The mean sCr level at day 17 (before group assignments) was significantly increased in group Nx versus group C, although the renal function was comparable before salt loading (Nx-LS vs. Nx-HS, 0.66 ± 0.04 mg/dL vs. 0.62 ± 0.05 mg/dL). After 2 weeks of salt loading, the mean sCr level at day 42 was significantly increased in group Nx-HS versus group Nx-LS, whereas no difference was noted between group C-LS and group C-HS (Fig. 1a). The mean SBP was significantly higher in group Nx than in group C; and among the group Nx subgroups, SBP was higher in group Nx-HS than in group Nx-LS (Fig. 1b). Mean BW was significantly reduced in group Nx versus group C (Fig. 1c). Figure 1d, e show the histological findings. Glomerulosclerosis and tubulointerstitial damage, such as tubular atrophy and interstitial fibrosis were observed in group Nx, with significantly more severe damage observed in group Nx-HS than in group Nx-LS.
Induction of high systolic blood pressure, renal damage and increased levels of intrarenal AngII by a high-salt diet in 5/6 nephrectomized rats. a Serum creatinine (sCr). b Systolic blood pressure (SBP). c Body weight. d Histopathological findings. Representative photographs of the glomeruli and tubulointerstitium. The upper row shows periodic acid–Schiff (PAS) staining (400×), while the lower row shows Masson’s trichrome (MT) staining (100×). e Glomerulosclerosis index. f Tubulointerstitial fibrotic area. g Representative immunostaining for intrarenal angiotensinogen (AGT) (400×) (upper row) and angiotensin II (AngII) (400×) (lower row). h Average immunoactivity of AGT and AngII in 20 microscopic fields for each slide was quantitatively evaluated with a semiautomatic image analysis system using Image J. Data are shown as mean ± standard deviation. **P < 0.01 versus C-LS; ##P < 0.01 versus C-HS; †P < 0.05 versus Nx-LS; ††P < 0.01 versus Nx-LS. C-HS, control rats receiving high-salt diet; C-LS, control rats receiving low-salt diet; Nx-HS, 5/6 nephrectomized rats receiving high-salt diet; Nx-LS, 5/6 nephrectomized rats receiving low-salt diet
Immunostaining for AGT and AngII revealed slight expression in the proximal tubular cells in group C and significantly increased expression in group Nx, especially group Nx-HS (Fig. 1g, h).
Increased levels of AngII in the SFO but not circulating AngII levels by HS diet in 5/6Nx rats
To evaluate intracerebral RAS activation, we performed immunohistochemical analyses of AGT and AngII in the SFO and compared the numbers of positive cells in the region of interest. The numbers of AGT- and AngII-positive cells were significantly increased in group Nx versus group C; furthermore, the number was dramatically increased in group Nx-HS versus group Nx-LS (Fig. 2a, b). These results indicate that AngII in the SFO was increased in CKD models and that further increased levels of AngII in the SFO were occured by only the HS diet in the CKD model. On the other hand, no significant intergroup differences were seen in levels of plasma AngII, a marker of circulating RAS activity.
Increased levels of AngII in the SFO but not circulating AngII by a high-salt diet in 5/6 nephrectomized rats. a Representative immunostaining for angiotensinogen (AGT) (200×) (upper row) and angiotensin II (AngII) (200×) (lower row) in the subfornical organ (SFO). High magnification (400×) is shown for Nx-high salt (HS). Examples of positive cells are indicated by black arrows. b Semiquantitative data of the number of AGT- and AngII-positive cells. c Plasma AngII concentration. Data are shown as mean ± standard deviation. **P < 0.01 versus C-LS; ##P < 0.01 versus C-HS; ††P < 0.01 versus Nx-LS. C-HS, control rats receiving high-salt diet; C-LS, control rats receiving low-salt diet; Nx-HS, 5/6 nephrectomized rats receiving high-salt diet; Nx-LS, 5/6 nephrectomized rats receiving low-salt diet
Exacerbation of sodium appetite by HS diet in 5/6Nx rats
In group C, the evaluated sodium appetite in the two-bottle test revealed no significant change between the C-LS and C-HS groups (Fig. 3). On the other hand, in the Nx group, the Nx-HS rats ingested significant amounts of saline compared to the Nx-LS rats. Furthermore, salt preference was also significantly higher in the Nx-HS versus Nx-LS group (32.73 ± 8.59% vs. 17.75 ± 5.50%, respectively; P < 0.01).
Exacerbation of sodium appetite by a high-salt diet in 5/6 nephrectomized rats. We evaluated sodium appetite using the two-bottle test (tap water and 0.9% saline). The test was conducted for 4 consecutive days, and the average daily intake of saline was calculated. During the test, the rats had ad libitum access to a low-salt diet provided in a feeding box separated from the bottles. Data are shown as mean ± standard deviation. *P < 0.05 versus C-LS; **P < 0.01 versus C-LS; #P < 0.05 C-HS versus Nx-LS and Nx-HS; ##P < 0.01 C-HS versus Nx-LS and Nx-HS; ††P < 0.01 Nx-LS versus Nx-HS. BW, body weight; C-HS, control rats receiving high-salt diet; C-LS, control rats receiving low-salt diet; Nx-LS, 5/6 nephrectomized rats receiving low-salt diet; Nx-HS, 5/6 nephrectomized rats receiving high-salt diet
Inefficacy of renal damage and increased levels of intrarenal AngII by ICV administration of ZD 7155 in Nx-HS rats
We performed a continuous ICV administration of ZD 7155 (AT1R antagonist) using an Alzet osmotic pump as described above. After confirming that the Alzet brain infusion kit was correctly inserted into the LV (Supplementary Fig. 2), we performed the following analysis.
In the Nx-HS-C rats administered ICV vehicle, SBP was as high as in the Nx-HS group rats in Protocol 1; in contrast, in Nx-HS-Z rats administered ZD 7155, SBP was significantly lower (Fig. 4b).
Inefficacy of renal damage and increased levels of intrarenal AngII by intracerebroventricular administration of ZD 7155 (angiotensin II type 1 receptor antagonist) and a high-salt diet in 5/6 nephrectomized rats. a Serum creatinine (sCr). b Systolic blood pressure (SBP). c Body weight. d Histopathological findings. Representative photographs of the glomeruli and tubulointerstitium. The upper row shows periodic acid–Schiff (PAS) staining (400×), while the lower row shows Masson’s trichrome (MT) staining (100×). e Glomerulosclerosis index. f Tubulointerstitial fibrotic area. g Representative immunostaining for intrarenal angiotensinogen (AGT) (400×) (upper row) and angiotensin II (AngII) (400×) (lower row). h The average immunoactivity of AGT and AngII in 20 microscopic fields for each slide was quantitatively evaluated with a semiautomatic image analysis system using Image J. Data are shown as mean ± standard deviation. *P < 0.05 versus Nx-HS-C. Nx-HS-C, 5/6 nephrectomized rats fed a high-salt diet and administered vehicle (0.9% saline) intracerebroventricularly; Nx-HS-Z, 5/6 nephrectomized rats fed a high-salt diet and administered ZD 7155 solution intracerebroventricularly
On the other hand, there was no significant difference in sCr and BW at day 45 or in histological findings such as glomerulosclerosis index and tubulointerstitial fibrotic area between the Nx-HS-C and Nx-HS-Z groups. In addition, no significant intergroup difference was observed in the AGT- and AngII-positive areas in the renal tubular region in the immunohistochemical analysis, representing intrarenal RAS.
Attenuation of increased levels of AngII in the SFO by ICV administration of ZD 7155 in Nx-HS rats
The number of AGT- and AngII-positive cells in the SFO was significantly decreased in the Nx-HS-Z versus Nx-HS-C group (Fig. 5). However, plasma AngII, an indicator of circulating RAS, was increased in the Nx-HS-Z group, consistent with previous studies [16]. Plasma Ang-(1-7) levels were also measured in protocol 2, and no significant differences were found between the two groups (Nx-HS-C vs. Nx-HS-Z: 146.07 ± 7.38 pg/mL vs. 149.03 ± 29.68 pg/mL; P = 0.77).
Attenuation of increased levels of AngII in the SFO by intracerebroventricular administration of ZD 7155 and a high-salt diet in 5/6 nephrectomized rats. a Representative immunostaining for angiotensinogen (AGT) (200×) (upper row) and angiotensin II (AngII) (200×) (lower row) in the subfornical organ (SFO). High magnification (400×) is shown for Nx-HS-C and Nx-HS-Z. Examples of positive cells are indicated by black arrows. b Semiquantitative data of the number of AGT- and AngII-positive cells. c Plasma AngII concentration. Data are shown as mean ± standard deviation. *P < 0.05 versus Nx-HS-C. Nx-HS-C, 5/6 nephrectomized rats fed a high-salt diet and administered intracerebroventricular vehicle (0.9% saline); Nx-HS-Z, 5/6 nephrectomized rats fed a high-salt diet and administered intracerebroventricular ZD 7155 solution
Attenuation of sodium appetite by ICV administration of ZD 7155 in Nx-HS rats
Saline intake did not change in the Nx-HS-C versus Nx-HS group, whereas it was significantly decreased in the Nx-HS-Z group (Fig. 6). In addition, salt preference was significantly lower in the Nx-HS-Z group (Nx-HS-C vs. Nx-HS-Z: 36.26 ± 9.12% vs. 26.12 ± 7.92%; P < 0.05).
Attenuation of sodium appetite by intracerebroventricular administration of ZD 7155 and a high-salt diet in 5/6 nephrectomized rats. We evaluated sodium appetite using the two-bottle test (tap water and 0.9% saline). The test was conducted for 4 consecutive days, and the average daily intake of saline was calculated. During the test, the rats had ad libitum access to a low-salt diet provided in a feeding box separated from the bottles. Data are shown as mean ± standard deviation. **P < 0.01 Nx-HS-C versus Nx-HS-Z. BW, body weight; Nx-HS-C, 5/6 nephrectomized rats fed a high-salt diet and administered intracerebroventricular vehicle (0.9% saline); Nx-HS-Z, 5/6 nephrectomized rats fed a high-salt diet and administered intracerebroventricular ZD 7155 solution
Discussion
In this study, Nx-HS rats showed increased levels of AngII in the SFO and sodium intake. Furthermore, the ICV administration of an AT1R antagonist reduced the increased sodium appetite by attenuating increased levels of AngII in the SFO. To our knowledge, this is the first report to confirm that sodium appetite was enhanced in a CKD model rat with increased levels of AngII levels in the SFO but not increased circulating AngII levels.
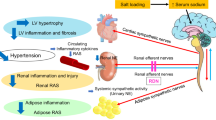
Intrarenal RAS induces local inflammation and fibrosis, playing a critical role in the pathophysiology of CKD and hypertension [28, 29]. Our group previously reported that intrarenal RAS was activated in 5/6Nx rats, resulting in renal fibrosis and kidney damage [18]. Thus, both circulating and organ-specific RAS are important for organ function and damage. Previous reports showing increased sodium appetite often used models in which circulatory RAS was activated [11,12,13,14], but in clinical practice, circulating RAS is not necessarily activated in patients with CKD. In the only report showing an increased sodium appetite in a CKD model rat, Gabriela et al. found that a renovascular hypertension model rat (two kidneys one clip) had circulating RAS activation and showed increased sodium intake after being subjected to water deprivation [4]. However, Cao et al. recently reported that, in Nx-HS rats, the circulatory RAS was not enhanced, whereas the intracerebral RAS in the forebrain nuclei such as the SFO was activated through a mechanism of reno-cerebral reflex mediated by the renal sympathetic nervous system [17]. We conducted this study with the idea that one of the reasons why patients with CKD often have difficulty adhering to salt restriction in clinical practice may be explained that excessive salt intake activates intracerebral RAS, forming a vicious cycle that leads to further salt intake.
In Nx-HS rats, plasma AngII levels, an indicator of circulating RAS, were decreased, but intrarenal RAS was inappropriately activated, accelerating renal damage through local inflammation and reactive oxygen species [17]. In this study, the Nx-HS group showed increased levels of the intrarenal AngII, increased SBP, and progression of glomerular sclerosis and tubulointerstitial fibrosis compared with the Nx-LS group, as reported previously. No intergroup differences in plasma AngII levels were noted, suggesting that circulating AngII and intrarenal AngII are independent in this model.
The numbers of AGT- and AngII-positive cells in the SFO were significantly increased in the Nx-HS versus Nx-LS and control groups. However, as mentioned above, the circulating AngII levels were not increased in the Nx-HS group, suggesting that AngII levels in the SFO are increased independently of circulating AngII. This mechanism was explained by Cao et al. in terms of the reno-cerebral reflex mediated by the renal sympathetic nervous system [17]. In this study, we also measured intrarenal norepinephrine concentration and immunohistochemical analyses (double staining with c-Fos and tyrosine hydroxylase) in the rostral ventrolateral medulla but found no evidence that the renal sympathetic nervous system and central nervous system were more active in the Nx-HS group than in the other groups (data not shown). This finding requires further verification in a future study.
To evaluate sodium appetite, we performed a two-bottle test during which all rats were given an LS diet and not allowed to consume any salt other than saline, their intake of which was evaluated. The increased saline intake in the Nx-HS versus Nx-LS group may be partly due to the rats growing accustomed to the taste of salt after being fed an HS diet for 2 weeks. However, in the C group, saline intake was only slightly increased in the C-HS versus C-LS group, suggesting that the excessive increase in saline intake was not merely a matter of taste acclimation but also the presence of pathological factors driving sodium appetite. In fact, the increased saline intake in the Nx-LS versus C-LS group indicated that sodium appetite was enhanced in this CKD model.
We hypothesized that intracerebral RAS activation was one of the main causes of the increased sodium appetite in the Nx-HS group. It is known that activated intracerebral RAS plays an important role in sodium appetite [5]; in particular, the SFO-vBNST pathway reportedly drove sodium appetite in an AT1R-dependent manner [7]. It was also reported that the deletion of AT1R in the SFO significantly attenuated sodium intake [8], while ICV administration of the AT1R antagonist losartan attenuated sodium appetite [4]. Therefore, we next performed a therapeutic intervention to suppress increased levels of AngII in the SFO. The continuous ICV administration of ZD 7155 (a strong AT1R antagonist) using an ALZET osmotic pump significantly reduced the number of AGT- and AngII-positive cells in the SFO, indicating the attenuation of increased levels of AngII in the SFO. In addition, sodium appetite was significantly reduced in the Nx-HS-Z versus Nx-HS-C group. On the other hand, plasma AngII levels were rather increased in the Nx-HS-Z group independent of AngII levels in the SFO. This suggests that the increased sodium appetite observed in Nx-HS was attenuated by the direct suppression of increased levels of AngII in the SFO. Moreover, because it was expected that under AT1R blockade in the Nx-HS-Z group, AngII may act through AT2R and may be degraded to Ang-(1-7) which may have inhibitory effect on sodium appetite, we measured plasma Ang-(1-7) levels in protocol 2. As mentioned in the results, no significant differences were found between the two groups, and the involvement of circulating Ang-(1-7) in the reduced sodium appetite in the Nx-HS-Z group was unclear in this study.
This study has several limitations. First, we measured AngII, the effector of RAS and AGT, the substrate of RAS to evaluate tissue-specific RAS in kidney and the SFO in this study. AngII is known to be an important activator of sodium appetite [5, 8], and AGT and AngII have been used to evaluate intrarenal RAS in previous studies [28, 29]. Therefore, we evaluated them in this experiment as well. Other RAS components will be evaluated in our future studies. Second, we could not evaluate renal sympathetic nerves in this study, so the mechanism of reno-cerebral reflux could not be verified. We also tried renal denervation in Nx-HS rats, but this resulted in an increased perioperative mortality rate, and no expected effect was observed, as seen in a previous study. Third, the mechanism in the brain was not examined because the main purpose of this study was to clarify whether sodium appetite is enhanced in CKD animal models. We will conduct further verification on this matter in the future. Fourth, it would have been helpful to have a normal salt diet group for control and Nx rats to evaluate the changes from low to high salt. Finally, in this study, the ICV administration of AT1R antagonist reduced SBP but did not attenuate increased levels of intrarenal AngII and renal damage, as in a previous study. This may be due to intrarenal AngII increase and renal damage aggravation due to HS loading were so strong that the dose of ZD 7155 used in this experiment was unable to ameliorate them. On the other hand, if the solubility of the ZD 7155 solution is exceeded, the catheter of the ALZET osmotic pump may become clogged, so the dosage of ZD 7155 could not be increased any further in this experiment. Further research is needed to verify the dosage and administration method to minimize renal damage, such as the weekly replacement of the ALZET osmotic pump or daily ICV bolus administration.
In conclusion, Nx-HS rats showed an enhanced sodium appetite, possibly due in part to increased levels of AngII in the SFO. The continuous ICV administration of ZD 7155 to directly inhibit increased levels of AngII in the SFO attenuated sodium appetite in this model. To our knowledge, few reports have shown an increase in sodium appetite in CKD model rats. It is also interesting that in the model of this study, the circulatory RAS indicated by plasma AngII levels was not enhanced, whereas the AngII in the SFO was increased. This may be an important factor that can scientifically explain why patients with CKD often have difficulty adhering to prescribed salt restrictions; however, further research is needed to verify the mechanism and explore methods that can be applied to clinical practice.
References
Cintia YP, Maria JC, Andre SM, Ximena EC, Jose A-R, Laura MV, et al. Molecular neurobiological markers in the onset of sodium appetite. Sci Rep. 2022;12:14224.
Joseph F, Robert MW, Shun-guang W, Alan KJ, Terry GB, Kathy Z, et al. Central mineral corticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H2241–H2251.
DiNicolantonio R, Hutchinson JS, Mendelsohn FA. Exaggerated salt appetite of spontaneously hypertensive rats is decreased by central angiotensin-converting enzyme blockade. Nature. 1982;298:846–8.
Gabriela ML, Jose VM, Eduardo C and Debora SAC. AngII and Aldosterone acting centrally participate in the enhanced sodium intake in water-deprived renovascular hypertensive rats. Front Pharmacol. 2021;12:679985.
Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2007;93:177–209.
Neil ER. Neurobehavioral mechanisms of sodium appetite. Nutrients. 2023;15:620.
Sarah SC, Andrew JL. The subfornical organ in sodium appetite: recent insights. Neuropharmacology 2018:107-113.
Matsuda T, Hiyama TY, Niimura F, Matsusaka T, Fukamizu A, Kobayashi K, et al. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat Neurosci. 2017;20:230–41.
Sunn N, McKinley M, Oldfield B. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J Neuroendocrinol. 2003;15:725–31.
Zardetto-Smith AM, Beltz TG, Johnson AK. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 1994;645:123–34.
Jalowiec JE. Sodium appetite elicited by furosemide: effects of differential dietary maintenance. Behav Biol. 1974;10:313–27.
Wagman W. Sodium chloride deprivation: development of sodium chloride as a reinforcement. Science. 1963;140:1403–4.
Stricker EM. Thirst and sodium appetite after colloid treatment in rats. J Comp Physiol Phychol. 1981;95:725-37.
Thunhorst RL, Fitts DA. Peripheral angiotensin causes salt appetite in rats. Am J Physiol. 1994;267:171–7.
Thunhorst RL, Beltz TG, Johnson AK. Effects of sunfornical organ lesions on acutely induced thirst and salt appetite. Am J Physiol. 1999;277:56–65.
Weisinger R, Denton D, Di Nicolantonio R, Hards D, McKinley M, Oldfield B. Subfornical organ lesion decreases sodium appetite in the sodium depleted rat. Brain Res. 1990;526:23–30.
Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, et al. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol. 2015;26:1619–33.
Ishigaki S, Ohashi N, Matsuyama T, Isobe S, Tsuji N, Iwakura T, et al. Melatonin ameliorates intrarenal renin-angiotensin system in a 5/6 nephrectomy rat model. Clin Exp Nephrol. 2018;22:539–49.
Roncari CF, Barbosa RM, Vendramini RC, De Luca LA Jr, Menani JV, Colombari E, et al. Enhanced Angiotensin II induced sodium appetite in renovascular hypertensive rats. Peptides. 2018;101:82–88.
Junggren IL, Zhao X, Sun X, Hedner T. Comparative cardiovascular effects of the angiotensin II type 1 receptor antagonists ZD 7155 and losartan in the rat. J Pharm Pharm. 1996;48:829–33.
Kondoh T, Matsunaga T. Intake and preference for dried bonito dashi in male Sprague-Dawley rats and C57BL/6 N mice. Physiol Behav. 2020;213:112708.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, et al. Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res. 2016;39:312–20.
Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, et al. Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Ren Physiol. 2008;295:1512–8.
Matsuyama T, Ohashi N, Aoki T, Ishigaki S, Isobe S, Sato T, et al. Circadian rhythm of the intrarenal renin-angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats. Hypertens Res. 2021;44:618–27.
Li HY, Hou FF, Zhang X, Chen PY, Liu SX, Feng JX, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol. 2007;18:528–38.
Miyazaki T, Aoyama I, Ise M, Seo H, Niwa T. An oral sorbent reduces overload of indoxyl sulphate and gene expression of TGF-beta1 in uraemic rat kidneys. Nephrol Dial Transpl. 2000;15:1773–81.
Zhou H, Kato A, Miyaji T, Yasuda H, Fujigaki Y, Yamamoto T, et al. Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol Dial Transpl. 2006;21:616–23.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharm Rev. 2007;59:251–7.
Navar LG, Harrison-Bernard LM, Nhishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22.
Acknowledgements
This study was supported by a grant from the Young Investigator Research Projects of Hamamatsu University School of Medicine in 2020 (awarded to Taro Aoki). Supplementary information is available at the Hypertension Research website.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aoki, T., Ohashi, N., Uchiyama, Y. et al. Sodium appetite is enhanced in 5/6 nephrectomized rat by high-sodium diet via increased levels of angiotensin II in the subfornical organ. Hypertens Res 48, 2595–2605 (2025). https://doi.org/10.1038/s41440-025-02289-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02289-7