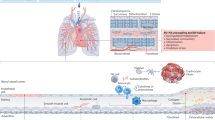
Abstract
Animal models of pulmonary arterial hypertension (PAH) demonstrate up-regulation of the renin angiotensin system. However, no clinical data are available to support the efficacy of angiotensin II inhibition in treatment for PAH. In this mini review, we will update the knowledge regarding regulation of the RAS system in PAH compared to primary hypertension in order to seek better treatments against PAH.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hassoun PM. Pulmonary arterial hypertension. N Engl J Med. 2021;385:2361–76.
Spiekerkoetter E, Kawut SM, de Jesus Perez VA. New and emerging therapies for pulmonary arterial hypertension. Annu Rev Med. 2019;70:45–59.
Vazquez ZGS, Klinger JR. Guidelines for the treatment of pulmonary arterial hypertension. Lung. 2020;198:581–96.
Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation. 2020;141:678–93.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022; https://doi.org/10.1183/13993003.00879-2022):2200879.
Yuan YM, Luo L, Guo Z, Yang M, Ye RS, Luo C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst. 2015;16:249–53.
Li G, Liu Y, Zhu Y, Liu A, Xu Y, Li X, et al. ACE2 activation confers endothelial protection and attenuates neointimal lesions in prevention of severe pulmonary arterial hypertension in rats. Lung. 2013;191:327–36.
Chen IC, Lin JY, Liu YC, Chai CY, Yeh JL, Hsu JH, et al. Angiotensin-Converting Enzyme 2 activator ameliorates severe pulmonary hypertension in a rat model of left pneumonectomy combined with VEGF inhibition. Front Med. 2021;8:619133.
Chassagne C, Eddahibi S, Adamy C, Rideau D, Marotte F, Dubois-Randé JL, et al. Modulation of angiotensin II receptor expression during development and regression of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2000;22:323–32.
Hu HH, Zhang RF, Dong LL, Chen EG, Ying KJ. Overexpression of ACE2 prevents hypoxia-induced pulmonary hypertension in rats by inhibiting proliferation and immigration of PASMCs. Eur Rev Med Pharm Sci. 2020;24:3968–80.
Hampl V, Herget J, Bíbová J, Baňasová A, Husková Z, Vaňourková Z, et al. Intrapulmonary activation of the angiotensin-converting enzyme type 2/angiotensin 1-7/G-protein-coupled Mas receptor axis attenuates pulmonary hypertension in Ren-2 transgenic rats exposed to chronic hypoxia. Physiol Res. 2015;64:25–38.
Bruce E, Shenoy V, Rathinasabapathy A, Espejo A, Horowitz A, Oswalt A, et al. Selective activation of angiotensin AT2 receptors attenuates progression of pulmonary hypertension and inhibits cardiopulmonary fibrosis. Br J Pharm. 2015;172:2219–31.
Burrell LM, Gayed D, Griggs K, Patel SK, Velkoska E. Adverse cardiac effects of exogenous angiotensin 1-7 in rats with subtotal nephrectomy are prevented by ACE inhibition. PLoS ONE. 2017;12:e0171975.
Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–6.
Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, et al. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Ren Physiol. 2006;291:F1308–14.
Takeda Y, Zhu A, Yoneda T, Usukura M, Takata H, Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2007;20:1119–24.
Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, et al. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept. 2011;166:90–7.
Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE, et al. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 2015;9:217–37.
Patel S, Hussain T. Dimerization of AT(2) and mas receptors in control of blood pressure. Curr Hypertens Rep. 2018;20:41.
Nair SK, Hersh EV, Margulies KB, Daniell H. Clinical studies in Myxomatous Mitral Valve Disease dogs: most prescribed ACEI inhibits ACE2 enzyme activity and ARB increases AngII pool in plasma. Hypertens Res. 2025;4:1477–90. https://doi.org/10.1038/s41440-025-02109-y.
Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313–26.
Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–72.
Sun X, Wang H, Hodge H, Wright KN, Ahmad S, Ferrario CM, et al. Amplifying effect of chronic lisinopril therapy on diastolic function and the angiotensin-(1-7) Axis by the G1 agonist in ovariectomized spontaneously hypertensive rats. Transl Res. 2021;235:62–76.
Iwanami J, Mogi M, Tsukuda K, Wang XL, Nakaoka H, Ohshima K, et al. Role of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis in the hypotensive effect of azilsartan. Hypertens Res. 2014;37:616–20.
Inaba S, Iwai M, Furuno M, Kanno H, Senba I, Okayama H, et al. Role of angiotensin-converting enzyme 2 in cardiac hypertrophy induced by nitric oxide synthase inhibition. J Hypertens. 2011;29:2236–45.
Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113–9.
Narula S, Yusuf S, Chong M, Ramasundarahettige C, Rangarajan S, Bangdiwala SI, et al. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–76.
Uri K, Fagyas M, Manyine Siket I, Kertesz A, Csanadi Z, Sandorfi G, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One. 2014;9:e87845.
Goldberg MR, Bradstreet TE, McWilliams EJ, Tanaka WK, Lipert S, Bjornsson TD, et al. Biochemical effects of losartan, a nonpeptide angiotensin II receptor antagonist, on the renin-angiotensin-aldosterone system in hypertensive patients. Hypertension. 1995;25:37–46.
Christen Y, Waeber B, Nussberger J, Porchet M, Borland RM, Lee RJ, et al. Oral administration of DuP 753, a specific angiotensin II receptor antagonist, to normal male volunteers. Inhibition of pressor response to exogenous angiotensin I and II. Circulation. 1991;83:1333–42.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–10.
Chen L, Kim SM, Eisner C, Oppermann M, Huang Y, Mizel D, et al. Stimulation of renin secretion by angiotensin II blockade is Gsalpha-dependent. J Am Soc Nephrol. 2010;21:986–92.
Agata J, Ura N, Yoshida H, Shinshi Y, Sasaki H, Hyakkoku M, et al. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens Res. 2006;29:865–74.
de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, Francois C, et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:780–9.
Montezano AC, Kuriakose J, Hood KY, Sin YY, Camargo LL, Namkung Y, et al. Ang-(1-7) and ET-1 Interplay Through Mas and ET(B) Receptor interaction defines a novel vasoprotective mechanism. Hypertension. 2025;82:267–81.
Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–8.
Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, et al. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–8.
Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, et al. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension. 2008;52:967–73.
Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, et al. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–40.
Dai HL, Guo Y, Guang XF, Xiao ZC, Zhang M, Yin XL. The changes of serum angiotensin-converting enzyme 2 in patients with pulmonary arterial hypertension due to congenital heart disease. Cardiology. 2013;124:208–12.
Li S, Wang Z, Yang X, Hu B, Huang Y, Fan S. Association between circulating angiotensin-converting enzyme 2 and cardiac remodeling in hypertensive patients. Peptides. 2017;90:63–8.
Emilsson V, Gudmundsson EF, Aspelund T, Jonsson BG, Gudjonsson A, Launer LJ, et al. Antihypertensive medication uses and serum ACE2 levels: ACEIs/ARBs treatment does not raise serum levels of ACE2. medRxiv. 2020. https://doi.org/10.1101/2020.05.21.20108738.
Úri K, Fagyas M, Kertész A, Borbély A, Jenei C, Bene O, et al. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316668435.
Itelman E, Segel MJ, Kuperstein R, Feinberg M, Segev A, Segal G, et al. Pulmonary hypertension is associated with systemic arterial hypertension among patients with normal left ventricular diastolic function. J Am Heart Assoc. 2021;10:e023603.
Dang Z, Su S, Jin G, Nan X, Ma L, Li Z, et al. Tsantan Sumtang attenuated chronic hypoxia-induced right ventricular structure remodeling and fibrosis by equilibrating local ACE-AngII-AT1R/ACE2-Ang1-7-Mas axis in rat. J Ethnopharmacol. 2020;250:112470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakayama, Y., Eguchi, S. The renin-angiotensin system in models of pulmonary arterial hypertension vs primary hypertension. Hypertens Res 48, 2522–2526 (2025). https://doi.org/10.1038/s41440-025-02299-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02299-5