Abstract
The rehabilitation of erectile function post-radical prostatectomy remains a great challenge in clinical practice. We aimed to develop a novel rat model suitable for investigating erectile dysfunction (ED) following nerve-sparing radical prostatectomy (NSRP) and to validate its applicability. Thirty-two male Sprague-Dawley rats were randomly assigned to undergo either a novel NSRP modeling procedure or sham surgery. The surgical protocol for the NSRP group included identification and dissection of the major pelvic ganglia and cavernous nerve, prostatectomy, and reconstruction of the lower urinary tract. Mean arterial pressure (MAP) and intracavernous pressure (ICP) were assessed at 1 and 2 weeks postoperatively. Subsequently, the penis tissues were collected for histopathological analyses. The results showed that rats in the NSRP group exhibited significantly lower maximum ICP/MAP ratios (0.39 ± 0.17 vs 0.77 ± 0.08, P < 0.0001) and areas under the ICP curve (2090.13 ± 1050.15 vs 4326.08 ± 1042.08, P = 0.0001) compared to the sham-operated group at 1 week postoperatively. By 2 weeks postoperatively, the NSRP group showed a significant improvement in both the maximum ICP/MAP ratio (0.73 ± 0.09 vs 0.39 ± 0.17, P < 0.0001) and the area under the ICP curve (3654.40 ± 679.28 vs 2090.13 ± 1050.15, P = 0.005) compared to the 1-week post-NSRP group. However, the area under the ICP curve in the NSRP group remained significantly lower than that in the sham-operated group at 2 weeks postoperatively (3654.40 ± 679.28 vs 5259.10 ± 951.48, P = 0.003). Similarly, histological staining suggested that smooth muscle atrophy and collagen deposition in the penis gradually improved over time post-NSRP. However, significant differences in the ratio of smooth muscle to collagen remained between the NSRP and sham-operated groups at 2 weeks postoperatively (0.22 ± 0.03 vs 0.27 ± 0.05, P = 0.011). In conclusion, this study established a novel rat model for investigating post-NSRP ED and validated its utility through erectile function monitoring and histological analysis. Further promising preclinical studies using this model are necessary to explore underlying mechanisms and evaluate the efficacy of treatments for post-NSRP ED.
Similar content being viewed by others
Introduction
Radical prostatectomy (RP) is regarded as the first-line treatment strategy for patients with localized prostate cancer [1]. Erectile dysfunction (ED) is a common complication post-RP [2]. Notwithstanding the optimization of nerve-sparing technique, nearly 40–53% of men encounter varying degrees of post-RP ED [3]. The rehabilitation of erectile function gradually increases with time post-RP, but the majority of patients experience a near complete loss of erectile function up to 3 months, with restoration to baseline being a rare occurrence [4, 5]. Hence, rehabilitation of erectile function post-RP remains a great challenge in clinical practice.
In general, injury to the pelvic plexus and neurovascular bundles during RP is widely recognized as the primary cause of post-RP ED [6, 7]. The cavernous nerve (CN), which originates from the pelvic plexus and course through the neurovascular bundles, plays a critical role in penile erection [6, 8]. Accordingly, rodent models of CN injury via crush, freezing, and excision of the CN have been developed for research on the pathophysiology of post-RP ED and evaluate potential therapeutic strategies [9,10,11]. In spite of promising outcomes observed in the rodent models, most of well-designed clinical trials have failed to confirm the lasting benefits of various treatments in improving the recovery of erectile function post-RP [12, 13]. Additionally, impaired erectile responses were also observed in rats, even in the absence of direct injury to the CN [14]. Despite existing rodent models, the precise mechanisms underlying ED post-RP, particularly post-nerve-sparing radical prostatectomy (NSRP), have yet to be elucidated.
A previous study demonstrated that a subset of periprostatic nerve fibers, distinct from the classical CN, also contributes to erectile function [15]. However, this anatomical feature has often been overlooked in existing rodent models of ED. Owing to the limited clinical application value of these models, it seems important to develop a novel animal model suitable for investigating post-NSRP ED. The optimal strategy is to accurately replicate the full NSRP procedure in rodents to enhance translational value. In this study, we developed a novel rat model that effectively addresses the technical challenges of identifying and preserving the CN and major pelvic ganglia (MPG), as well as performing bladder neck dissection and urinary tract reconstruction through bladder-to-urethra anastomosis. Evaluation of erectile function post-NSRP and histopathological analysis validate the applicability of this model for further research on post-NSRP ED.
Methods
Animals and ethical approval
Thirty-two 12-week-old male Sprague-Dawley rats were obtained from the Laboratory Animal Center of Xi’an Jiaotong University. All rats were housed in a standard laboratory environment with free access to food and water under a constant temperature (23 ± 1 °C) and a 12/12 h light/dark cycle. The rats were randomly divided into 2 groups received sham surgery (n = 16) or NSRP (n = 16). Each group was subsequently subdivided into two subgroups, with erectile function assessments conducted at 1 week and 2 weeks postoperatively. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health) [16], and were approved by the Biomedical Ethics Committee of Health Science Center of Xi’an Jiaotong University (No. XJTUAE20-1863).
Preoperative preparation
The rat was anaesthetized by intraperitoneal injection of 3% sodium pentobarbital (45 mg/kg), followed by intraperitoneal injection of cefoperazone-sulbactam sodium (10 mg/kg) to prevent infection, and subcutaneous injection of meloxicam (1 mg/kg) to relieve pain half an hour before surgery. Anesthesia adequacy was confirmed by the absence of significant responses to painful stimuli, and all efforts were made to minimize suffering in the rat. Once adequate anesthesia was achieved, the lower abdominal hair was shaved and disinfected with iodophor.
Surgical procedures
Identification and dissection of the CN and MPG
-
I.
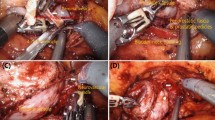
The rat was fixed in the supine position, and a 2–3 cm longitudinal, midline incision was made in the lower abdomen (Fig. 1A). The surgical field was adequately exposed using an abdominal retractor. All procedures were performed under surgical loupe magnification and optimal illumination. The retropubic space was bluntly dissected and exposed, and the peritoneal contents were gently displaced toward the upper abdomen using a sterile cotton swab. After removing the periprostatic and pelvic fat, the prostate was gently displaced medially with a cotton swab to clearly expose the MPG and CN (Fig. 1B).
Fig. 1: NSRP procedure in the rat. A Creation of a 2–3 cm midline lower abdominal incision following shaving and disinfection. B Identification of the CN, PN, and MPG. C Dissection of the CN and MPG. D–F Dissection, transection, and closure of the bladder neck. G, H Dissection and transection of the bilateral vas deferens. I, J Exposure of the bilateral seminal vesicles through the space between the prostate and bladder. K Dissection and transection of the prostatic branches originating from the MPG or CN. L, M Exposure of the prostate apex and the anterior wall of the rectum. N Excision of the prostate and seminal vesicles. O Insertion of a PE-50 tube into the posterior urethra. P Creation of a 0.5 cm incision in the anterior bladder wall. Q Performance of urethrovesical anastomosis. R Verification of the integrity of the CN and MPG. NSRP nerve-sparing radical prostatectomy, CN cavernous nerve, PN pelvic nerve, MPG major pelvic ganglia.
-
II.
A longitudinal incision was made along the course of CN through the inner pelvic fascia, extending from the ureter-bladder junction to the prostatic urethra junction. Gentle blunt dissection of the MPG and CN from the surface of the prostate was performed using ophthalmic forceps (Fig. 1C).
-
III.
The prostate was carefully separated from the bladder to fully expose the bladder neck (Fig. 1D). The bladder neck was then transected and sutured using 6-0 absorbable sutures (Fig. 1E, F). Since the prostate received its blood supply from vessels located on both sides of the bladder neck, particular care was taken to avoid injury to these vessels in order to prevent severe bleeding.
-
IV.
After transection of the bladder neck, the bladder was retracted toward the upper abdomen. The bilateral seminal vesicles and vas deferens were then carefully dissected posterior to the bladder (Fig. 1G). Both vas deferens were transected, and the seminal vesicles were gently mobilized from the space between the bladder and prostate (Fig. 1H–J).
-
V.
The branches extending from the MPG or CN to the prostate were carefully identified and dissected, and then sequentially transected to fully mobilize the bilateral MPG and CN from the prostate and seminal vesicles (Fig. 1K).
Prostatectomy and lower urinary tract reconstruction
-
I.
The prostatic apex and membranous urethra were fully exposed. The posterior fascia between the prostate and rectum was then carefully incised (Fig. 1L), allowing complete mobilization of the prostate from the rectum (Fig. 1M).
-
II.
The urethra at the prostatic apex was sharply transected via surgical scissors, enabling complete removal of the prostate and seminal vesicles from the pelvic cavity (Fig. 1N).
-
III.
Subsequently, a PE-50 tube (approximately 1.5 cm in length) was inserted into the posterior urethra (Fig. 1O). A longitudinal incision (approximately 0.5 cm in length) was made on the anterior wall of the bladder, just above the bladder neck (Fig. 1P). Continuous suturing with 6-0 nylon thread was employed to anastomose the bladder mucosa to the urethral mucosa. After completing the posterior segment of the anastomosis, the PE-50 tube was inserted into the bladder, and the anterior segment of the bladder-to-urethra anastomosis was performed (Fig. 1Q). Typically, 6–8 sutures were required to ensure a secure anastomosis. The preserved CN and MPG were then carefully examined to confirm the absence of iatrogenic injury (Fig. 1R). Finally, the abdominal incision was closed with continuous sutures and disinfected with iodophor.
In the sham-operated group, rats underwent the same anesthetic procedure and preliminary surgical steps as described above. After brief exposure of the MPG and CN, the abdominal incision was closed.
Postoperative care
The rat was placed on a heating pad for 1 h immediately following the operation to maintain body temperature. Four hours after recovery from anesthesia, food and water were provided. Postoperative care consisted of daily intraperitoneal injections of cefoperazone-sulbactam sodium (10 mg/kg) and subcutaneous injections of meloxicam (1 mg/kg) for three consecutive days. At the end of the experiment, all rats were euthanized via an overdose of anesthesia.
Erectile function monitoring
Rats in the sham-operated and NSRP groups were intraperitoneally administered 3% sodium pentobarbital (45 mg/kg) at 1 and 2 weeks postoperatively, respectively. Following anesthesia, blood pressure and intracavernous pressure (ICP) catheters were inserted according to the established protocol [17]. The MPG and CN were fully exposed, and electrical stimulation (5 V, 15 Hz, 60 s duration) was applied to the CN with a 5-min interval between stimulations, for a total of three stimulations. Mean arterial pressure (MAP), baseline ICP, maximum ICP, maximum ICP/MAP ratio, and the area under the ICP curve were measured using the BL-420S biological signal acquisition and analysis system (Chengdu Techman Software Co., Ltd., Sichuan, China).
Masson trichrome staining and immunohistochemistry
The penis tissues were harvested and fixed in 4% paraformaldehyde for 24 h, dehydrated by an ethanol gradient, and then embedded in paraffin. The 5 μm thick paraffin-embedded sections were subjected to dewaxing, rehydration, and stained using a Masson trichrome staining kit (G1006, Servicebio, Wuhan, China) according to the manufacturer’s instruction. Image J software was employed to analyze Masson’s staining results.
For immunohistochemistry analysis, the paraffin sections were dewaxed, hydrated, antigen repaired following standard protocol [18]. The sections were then treated with 3% H2O2 for 20 min and blocked with 3% bovine serum albumin for 30 min, and then incubated at 4 °C with alpha smooth muscle actin (α-SMA, 1:2000, ab124964, abcam, Cambridge, UK) primary antibodies overnight followed by anti-rabbit secondary antibody (G1213, 1:200, Servicebio, Wuhan, China) for 1 h at room temperature. Finally, the sections were treated with diaminobenzidine (DAB) chromogenic kit (G1212, Servicebio, Wuhan, China) and counterstained with hematoxylin.
Statistical analysis
Data analysis was conducted using GraphPad Prism9 (version 9.5.1, San Diego, California, USA), and quantitative data were expressed as means± standard deviation. Following the Shapiro–Wilk normality test, one-way analysis of variance (ANOVA) was used to compare means across multiple groups, followed by the LSD test for pairwise comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
Evaluation of erectile function and confirmation of NSRP model
Sixteen rats underwent NSRP, with two death postoperatively due to hydronephrosis resulting from anastomotic stenosis. The mean duration of the surgical procedure in the NSRP group was 42.4 ± 2.8 min. The durations of individual surgical steps are detailed in Supplementary Table 1. All rats in the sham-operated group survived until the end of the experiment.
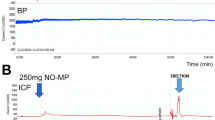
To evaluate the recovery of erectile function in the NSRP rat model, ICP-related parameters were measured, as shown in Table 1 and Fig. 2. At 1 week postoperatively, rats in the NSRP group exhibited significantly lower maximum ICP/MAP ratios (0.39 ± 0.17 vs 0.77 ± 0.08, P < 0.0001) (Fig. 2B) and areas under the ICP curve (2090.13 ± 1050.15 vs 4326.08 ± 1042.08, P = 0.0001) (Fig. 2C) compared to the sham-operated group. By 2 weeks postoperatively, the NSRP group showed a significant improvement in both the maximum ICP/MAP ratio (0.73 ± 0.09 vs 0.39 ± 0.17, P < 0.0001) (Fig. 2B) and the area under the ICP curve (3654.40 ± 679.28 vs 2090.13 ± 1050.15, P = 0.005) (Fig. 2C) compared with the 1-week post-NSRP group. However, the area under the ICP curve in the NSRP group remained significantly lower than that in the sham-operated group at 2 weeks postoperatively (3654.40 ± 679.28 vs 5259.10 ± 951.48, P = 0.003) (Fig. 2C).
A. Representative ICP and MAP responses in the NSRP group and sham-operated group. The erectile function was evaluated by (B) maximum ICP/MAP and (C) AUC for ICP. Sham-operated (n = 8), NSRP (n = 7). Data were expressed as Mean ± SD. One-way ANOVA followed by LSD test for multiple comparison. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns not significant. ICP intracavernous pressure, MAP mean arterial pressure, CN cavernous nerve, Post-op postoperative, NSRP nerve-sparing radical prostatectomy, AUC area under the curve, SD standard deviation.
Histological analysis of smooth muscle and collagen of penis
To evaluate pathological alterations following NSRP, Masson trichrome staining was performed (Fig. 3A). The result showed that at 1-week post-operation, the NSRP group exhibited a significant decrease in smooth muscle to collagen ratio compared to the sham-operated group (0.17 ± 0.02 vs 0.22 ± 0.02, P = 0.03). Moreover, a significant improvement in the smooth muscle to collagen ratio was observed in the NSRP group from postoperative week 1 to week 2 (0.17 ± 0.02 vs 0.22 ± 0.03, P = 0.045). However, the ratio of smooth muscle to collagen in the NSRP group remained significantly lower compared to that in the sham-operated group at 2 weeks postoperatively (0.22 ± 0.03 vs 0.27 ± 0.05, P = 0.011) (Fig. 3B). The same trends were also observed by immunohistochemical staining for α-SMA (Fig. 3C). In a word, the aforementioned results suggested that smooth muscle atrophy and collagen deposition improved over time but were not entirely normal.
A Representative images of Masson staining of corpus cavernosum. B Semiquantitative image analysis of the ratio of smooth muscle to collagen (n = 6 per group). C Representative images of immunohistochemical staining for α-SMA. Data were expressed as Mean ± SD. One-way ANOVA followed by LSD test for multiple comparison. *P < 0.05. NSRP nerve-sparing radical prostatectomy, α-SMA alpha smooth muscle actin, Post-op postoperative, SD standard deviation.
Discussion
The outcomes of RP are commonly described as the “trifecta” comprising urinary continence, sexual function, and oncological control [19, 20]. The advancement of surgical techniques has led to notable strides in the management of prostate cancer [21]. However, an ideal treatment strategy to facilitate the recovery of erectile function post-RP remains undetermined. Reported rates of post-NSRP ED range from 10 to 46% at 1 year and from 6 to 37% at 2 years postoperatively [22]. Variations in patient selection and the application of nerve preservation techniques may significantly influence the recovery of erectile function. Moreover, even with successful nerve preservation, concomitant neuropraxia is inevitable due to compromised vascular supply and loss of supporting tissue [23, 24].
Currently, a substantial portion of our understanding of the pathophysiological mechanisms underlying ED, as well as the advancement of related therapeutic strategies, has been derived from animal studies. Quinlan et al. were the first to show that electrical stimulation of the CN in Sprague-Dawley rats induced sustained penile erection, which positioned rodents as the preferred model for investigating erectile function, supplanting other larger animals [10]. The rat model has since been further developed and optimized. The application of the maximum ICP/MAP ratio offered a more objective and quantitative method for evaluating erectile function in rodent models, particularly in rats [17, 25]. Bilateral CN injury in rats is widely recognized as the standard experimental model for studying post-RP ED [26]. However, the variability in model construction methods and the lack of consensus on standardized approaches often lead to inconsistent and conflicting experimental results, making direct comparisons challenging. Moreover, the ancillary penile nerves originating from the MPG complement the CN in autonomic innervation of the penis and account for 45% of the ICP response to electrical stimulation of the medial preoptic area [27]. In other words, besides the CN, other nerve fibers originating from the MPG also play a significant and non-negligible role in erectile function. Therefore, when utilizing rat models to investigate the pathophysiological mechanisms underlying post-RP ED, it is crucial to consider the potential physiological significance and value of these ancillary penile nerves.
To enhance the clinical applicability of experimental results obtained from rat models, the optimal approach is to align surgical procedures closely with those performed in clinical settings. Naturally, this presents certain technical challenges in animal experiments. In light of these challenges, we provided a detailed description of the modeling methodology and successfully developed a novel rat model to study post-RP ED in this study. Monitoring postoperative erectile function in rats from the NSRP group indicated a significant decline in erectile function at 1 week postoperatively, followed by a marked improvement at 2 weeks. This pattern was consistent with the trend of erectile recovery observed in most clinical patients [28]. Histological analysis revealed notable smooth muscle atrophy and collagen deposition in the corpus cavernosum post-NSRP. The pathological changes exhibited partial alleviation over time, though the extent remained relatively limited. Indeed, the reasons for post-RP ED are multifaceted and complex, including cavernous nerve injury, inadequate blood supply, lack of oxygenation in the penile cavernosum, neuropraxia-induced damage to erectile tissue leading to veno-occlusive dysfunction, or a combination of these etiologies [6, 29, 30]. Notably, unlike previous rat models that directly injure the CN, our model preserves both the CN and MPG, more accurately mimicking the clinical surgical scenario. This allows for a more comprehensive investigation of potential mechanisms influencing erectile function recovery post-NSRP, including the role of ancillary penile nerves. It is worth noting, however, that the surgical technique used to establish this model may require time and experience to master. Furthermore, as reported, penile fibrotic changes occurred as early as two months postoperatively [31]. Early improvement of penile fibrosis is crucial for the rehabilitation of erectile function post-RP. Thus, research on therapeutic approaches to promote erectile function recovery based on our established NSRP rat model may represent a promising direction.
Although this novel rat model offers valuable insights, it still has certain limitations. First, the rats used for modeling were young, which did not align with the older age of patients undergoing NSRP. Second, patients often have comorbidities such as hypertension and diabetes, which could significantly affect postoperative recovery of erectile function. However, these factors were not assessed in the healthy rats used in the model. Third, the relatively small sample size may somewhat limit the robustness of our conclusions. Further studies with larger cohorts are warranted to confirm these findings.
Conclusion
This study presented the development of a novel rat model for investigating post-NSRP ED and validated its utility through erectile function monitoring and histological analysis. Further promising preclinical studies using this model are necessary to explore underlying mechanisms and evaluate the efficacy of treatment strategies for post-NSRP ED.
Data availability
All data generated or analyzed in this study are available from the corresponding author upon reasonable request.
References
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
Wallis CJD, Glaser A, Hu JC, Huland H, Lawrentschuk N, Moon D, et al. Survival and complications following surgery and radiation for localized prostate cancer: an international collaborative review. Eur Urol. 2018;73:11–20.
Tal R, Alphs HH, Krebs P, Nelson CJ, Mulhall JP. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med. 2009;6:2538–46.
Katz D, Bennett NE, Stasi J, Eastham JA, Guillonneau BD, Scardino PT, et al. Chronology of erectile function in patients with early functional erections following radical prostatectomy. J Sex Med. 2010;7:803–9.
Nelson CJ, Scardino PT, Eastham JA, Mulhall JP. Back to baseline: erectile function recovery after radical prostatectomy from the patients’ perspective. J Sex Med. 2013;10:1636–43.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7.
Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–85.
Awad A, Alsaid B, Bessede T, Droupy S, Benoît G. Evolution in the concept of erection anatomy. Surg Radiol Anat. 2011;33:301–12.
Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, Lue TF. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470–5.
Quinlan DM, Nelson RJ, Partin AW, Mostwin JL, Walsh PC. The rat as a model for the study of penile erection. J Urol. 1989;141:656–61.
El-Sakka AI, Hassan MU, Selph C, Perinchery G, Dahiya R, Lue TF. Effect of cavernous nerve freezing on protein and gene expression of nitric oxide synthase in the rat penis and pelvic ganglia. J Urol. 1998;160:2245–52.
Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–86.
Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47.
Yamashita S, Kato R, Kobayashi K, Hisasue S, Arai Y, Tsukamoto T. Nerve injury-related erectile dysfunction following nerve-sparing radical prostatectomy: a novel experimental dissection model. Int J Urol. 2009;16:905–11.
Zvara P, Spiess PE, Merlin SL, Bégin LR, Brock GB. Neurogenic erectile dysfunction: the course of nicotinamide adenine dinucleotide phosphate diaphorase-positive nerve fibers on the surface of the prostate. Urology. 1996;47:146–51.
Research ILA, Sciences CL, Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996.
Martínez-Piñeiro L, Brock G, Trigo-Rocha F, Hsu GL, Lue TF, Tanagho EA. Rat model for the study of penile erection: pharmacologic and electrical-stimulation parameters. Eur Urol. 1994;25:62–70.
Kim SW, Roh J, Park CS. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50:411–8.
Salomon L, Saint F, Anastasiadis AG, Sebe P, Chopin D, Abbou CC. Combined reporting of cancer control and functional results of radical prostatectomy. Eur Urol. 2003;44:656–60.
Walsh PC. The status of radical prostatectomy in the United States in 1993: where do we go from here? J Urol. 1994;152:1816.
Costello AJ. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat Rev Urol. 2020;17:177–88.
Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–30.
Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy. Urology. 2003;61:491–7.
Ganzer R, Blana A, Gaumann A, Stolzenburg J-U, Rabenalt R, Bach T, et al. Topographical anatomy of periprostatic and capsular nerves: quantification and computerised planimetry. Eur Urol. 2008;54:353–61.
Chen KK, Chan JY, Chang LS, Chen MT, Chan SH. Intracavernous pressure as an experimental index in a rat model for the evaluation of penile erection. J Urol. 1992;147:1124–8.
Chung E, De Young L, Brock GB. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8:3291–305.
Sato Y, Rehman J, Santizo C, Melman A, Christ GJ. Significant physiological roles of ancillary penile nerves on increase in intracavernous pressure in rats: experiments using electrical stimulation of the medial preoptic area. Int J Impot Res. 2001;13:82–88.
Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701–5.
Breza J, Aboseif SR, Orvis BR, Lue TF, Tanagho EA. Detailed anatomy of penile neurovascular structures: surgical significance. J Urol. 1989;141:437–43.
Müller A, Tal R, Donohue JF, Akin-Olugbade Y, Kobylarz K, Paduch D, et al. The effect of hyperbaric oxygen therapy on erectile function recovery in a rat cavernous nerve injury model. J Sex Med. 2008;5:562–70.
Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–6.
Author information
Authors and Affiliations
Contributions
YG, RT, and DH designed the study. YG and RT performed the experiments. DJ, ZR, HF, XX, and GL collected the samples and recorded the data. YG, RT, and ZW analyzed the data. YG and RT drafted the manuscript. LY and DH revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Y., Tao, R., Wang, Z. et al. A novel rat model for investigating erectile function after nerve-sparing radical prostatectomy. Int J Impot Res (2025). https://doi.org/10.1038/s41443-025-01142-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-025-01142-2
This article is cited by
-
Comment on: A novel rat model for investigating erectile function after nerve-sparing radical prostatectomy
International Journal of Impotence Research (2025)