Abstract
The growth of lamellar crystals has been studied in particular for spherulites in polymeric materials. Even though such spherulitic structures and their growth are of crucial importance for the mechanical and optical properties of the resulting polymeric materials, several issues regarding the residual stress remain unresolved in the wider context of crystal growth. To gain further insight into micro-mechanical forces during the crystallization process of lamellar crystals in polymeric materials, herein, we introduce tetraarylsuccinonitrile (TASN), which generates relatively stable radicals with yellow fluorescence upon homolytic cleavage at the central C–C bond in response to mechanical stress, into crystalline polymers. The obtained crystalline polymers with TASN at the center of the polymer chain allow not only to visualize the stress arising from micro-mechanical forces during polymer crystallization via fluorescence microscopy but also to evaluate the micro-mechanical forces upon growing polymer lamellar crystals by electron paramagnetic resonance, which is able to detect the radicals generated during polymer crystallization.
Similar content being viewed by others
Introduction
Crystalline polymers such as polyethylene and polyethylene terephthalate are indispensable for our daily lives. Their mechanical and optical properties strongly depend on their crystallinity1, which in turn is closely correlated to molding parameters. The crystallization of such synthetic polymers has been extensively studied, especially from a morphological and kinetic perspective2. Unlike amorphous resins, crystalline polymers experience stress at freezing and contain residual stress due to crystallization. However, the micro-mechanical forces during polymer crystallization remains an intensely debated topic, since suitable techniques to quantitatively evaluate this phenomenon have not yet been established. So far, only theoretical, indirect-experimental, or empirical discussions have been employed. In order to avoid material failure due to micro-mechanical forces during polymer crystallization, researchers and manufacturers have employed various experimental techniques. The approaches to assess the magnitude of micro-mechanical forces during polymer crystallization in polymer components can be classified into three distinct categories: Non-destructive3, destructive4, and predictive5. Among these, non-destructive methods such as holographic interferometry and synchrotron X-ray diffraction analysis have been used to visualize the physical relaxation of components during heating and to calculate their initial stress state. Despite the fact that the non-destructive nature of these methods is without doubt an advantage, they have been unable to accomplish neither a qualitative evaluation nor stress visualization in real time. From the viewpoint of the molecular topology of the amorphous phase, micro-mechanical forces during polymer crystallization in tie chain segments, which are molecular connections between individual crystallites, have been yet an important issue for the tensile or compression behavior of semicrystalline polymers, the fracture toughness, and the resistance to slow crack growth6,7. Topological features such as tie chain segments and loop segments, and the alternation of crystalline and amorphous domains have formed the basis of most molecular-level description of the semicrystalline state8,9.
On the other hand, various types of mechano-functional polymers have recently been developed based on mechano-responsive molecules called mechanophores10,11,12, which are activated in response to mechanical stress arising from exposure to ultrasonic13,14,15,16, stretching17,18,19, compression20, swelling21, or solvent-coagulation forces22,23,24,25. Our group has been developing radical-type mechanochromophores that exhibit coloration and fluorescence derived from relatively stable radicals generated upon homolytic cleavage of C–C bonds in response to exposure to mechanical stress. For example, a diarylbibenzofuranone22 generates blue radicals upon the homolytic cleavage of its central C–C bond, while tetraarylsuccinonitrile (TASN; Fig. 1a)26 generates pink radicals that emit yellow fluorescence under UV irradiation.
a Chemical structure of tetraarylsuccinonitrile (TASN) and its equilibrium with the corresponding C–C-cleaved radicals. b Schematic image of the crystallization of a TASN-centered polymer from its molten state. c Synthetic scheme of TASN-containing linear polycaprolactone (L-PCL), TASN-containing star polycaprolactone (S-PCL), and control polycaprolactones that contain bisphenol A (C1-PCL) or fluorescein (C2-PCL) at the center of the chain.
In the present study, the above-mentioned radical-type mechanochromophores, especially TASN, were introduced into the center of the main chain of crystalline polymers. As TASN at the cross-linking point can be cleaved in response to the solvent-coagulation force in freezing cross-linked polymer gels, we hypothesized that TASN in a tie molecule could potentially be mechanically cleaved by the growth of lamellar crystals (henceforth: crystallization-induced mechanofluorescence (CIMF)) (Fig. 1b). Accordingly, the corresponding fluorescence of radicals generated from TASN would enable a visualization of the crystallization. The advantage of using radical-type mechanochromophores lies in the generated radicals, which can be examined by electron paramagnetic resonance (EPR) measurements. Thus, the present system is suitable for the detection of low degrees of stress that arise from micro-mechanical forces during polymer crystallization. Fluorescence microscopy observations of CIMF allows the precise identification of the stress location and an in-depth clarification of the polymer crystallization process.
Results
Strategy and system
In this study, polycaprolactone (PCL) was chosen as the crystalline polymer based on the following three considerations. Firstly, even at room temperature, TASN is in equilibrium with a small amount of dissociated radicals, and it is unstable at temperatures beyond 100 °C. The melting point (~50 °C) and the crystallization temperature (~30 °C) of PCL mean that TASN can be used under relatively mild conditions, which the effect of this paper is limited to PCL melts. Secondly, a series of PCLs can be easily synthesized in one step using the living ring-opening polymerization of ε-caprolactone initiated by TASN derivatives with two (TASN-diol) or four hydroxy groups (TASN-tetraol) as the initiators. Thirdly, PCL has been studied extensively and ample basic data on PCL is available27,28,29,30,31,32,33,34,35.
As shown in Fig. 1c, a series of PCLs were synthesized using (1) TASN-diol, (2) TASN-tetraol, (3) Bisphenol A-diol, and (4) Fluorescein-diol as initiators, in the presence of diphenyl phosphate (DPP) as the bulk catalyst36,37. Linear and star-shaped PCLs with TASN are henceforth referred to as L-PCL and S-PCL, respectively. Control PCLs prepared from Bisphenol A-diol and Fluorescein-diol are henceforth referred to as C1-PCL and C2-PCL, respectively. C1-PCL was synthesized as a control PCL that is not cleaved in response to micro-mechanical forces during polymer crystallization, while C2-PCL was synthesized as a polymer that continuously emits fluorescence during the formation of spherulites. All PCLs were successfully synthesized and their molecular weight, molecular weight distribution, and thermal properties are summarized in Table 1. PCLs with different molecular weights were successfully synthesized by changing the monomer-to-initiator feed ratio.
CIMF during isothermal crystallization
CIMF was investigated on the series of synthesized PCLs. Initially, the PCL samples were exposed to simple heating. During the heating process (heating rate: 50 °C min−1), the PCL samples were completely molten at 70 °C, where they were kept for 2 min. Subsequently, the samples were cooled to 30 °C (cooling rate: –20 °C min−1), where they were kept for crystallization. During the crystallization, both pink coloration and yellow fluorescence, due to the dissociation of TASN, were observed (Fig. 2a). During the cooling process from 70 °C to 0 °C, EPR measurements on L-PCL (Mn,NMR = 13,000) supported the hypothesis that the dissociation of TASN is mainly induced by crystallization and not by melting (Supplementary Fig. 21). Solid-state UV-vis (Fig. 2b) and fluorescence measurements (Fig. 2c) on L-PCL (Mn,NMR = 13,000) after isothermal crystallization showed an absorption peak at 548 nm in the UV-vis measurement and an emission peak at 560 nm in the fluorescence measurement. These peaks unambiguously indicate the generation of diarylacetonitrile (DAAN) radicals derived from TASN26. Furthermore, in the solid-state fluorescence measurement, the fluorescence intensity drastically increased upon shifting the excitation wavelength to 548 nm. Therefore, an argon laser (λex = 514 nm) was chosen for the subsequent fluorescent microscopy observations of PCLs in order to gain clear images of the spherulites.
a CIMF of L-PCL (Mn,NMR = 13000), which contains a TASN moiety at the center of the main chain. b Solid-state UV-vis spectrum of L-PCL (Mn,NMR = 13,000) after isothermal crystallization. c Solid-state fluorescence spectra of L-PCL (Mn,NMR = 13,000) after isothermal crystallization under different excitation wavelength. (DAAN radical: diarylacetonitrile radical derived from TASN).
Quantitative evaluation of the amount of radicals generated during CIMF
The generation of radicals during the isothermal crystallization was examined for the L-PCLs and S-PCLs. In the present system, the mechanical force induced by crystallization can be evaluated by EPR measurements of the generated radicals. We speculate that the knowledge of unpaired spin density may allow quantitative estimates of segmental forces by assuming that the dissociated radicals from TASN moieties can be detected without oxidation, which is controlled by the sealing of the capillary after being degassed. The concentration of the radicals formed from the cleavage of TASN was determined by comparing the area of the observed integral spectrum with a 0.01-mM solution of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) in benzene under the same experimental conditions. The PCL samples were fully molten at 70 °C, and subsequently cooled to 30 °C (cooling rate: –20 °C min−1) for crystallization. Figure 3a, b show the EPR spectra of L-PCL (Mn = 24,400) and S-PCL (Mn = 25,200), respectively, during the crystallization at 30 °C. The calculated g value (2.003) in both cases indicates that the generated radicals are carbon-centered. The signal intensity generated from TASN increases with time. Fig. 3c, d show the dissociation ratios of TASN calculated from the EPR spectra in Fig. 3a, b, respectively. The TASN dissociation ratio reached an equilibrium after 90 minutes for L-PCL and after 330 min for S-PCL. It should be noted that the time to reach the equilibrium approximately doubled when the molecular weight doubled38,39,40,41.
EPR spectra of (a) L-PCL (Mn,NMR = 24400) and (b) S-PCL (Mn,NMR = 25,200) during isothermal crystallization. Dissociated TASN (%) in (c) L-PCL (Mn,NMR = 7100: black circle, 13,000: red square, and 24,400: blue triangle) and (d) S-PCL (Mn,NMR = 12,600: black circle, and 25,200: red square) during isothermal crystallization. e Relationship between molecular weight and dissociated TASN (%) of L-PCL (Mn,NMR = 7100, 13,000, and 24,400; blue circle and line) and S-PCL (Mn,NMR = 12,600 and 25,200; red square and line) during isothermal crystallization. f Linear relationship between the molecular weight of the arm and dissociated TASN (%) of L-PCL (Mn,NMR = 7100, 13,000, and 24,400; blue circle and line) and S-PCL (Mn,NMR = 12,600 and 25,200; red square and line) during isothermal crystallization. Plausible schematic illustration of chain conformations of (g) high- and low-molecular-weight polymers and (h) linear and star-shaped crystalline polymers between lamellar structures.
Figure 3e, f show the plots of the dissociation ratios of the TASN moiety for the PCL samples as a function of the molecular weight and molecular weight of the arm, respectively. These data suggest (1) an increase in the dissociation ratio of the TASN moiety with increasing molecular weight and (2) a large difference in the dissociation ratio of the TASN moiety due to the difference in primary structures (linear or star-shaped polymers). A plausible mechanism to rationalize the experimentally observed results could be based on the following considerations: (1) with increasing molecular weight in the same crystallinity (Table 1), the fraction of TASN moieties between crystallites6,7,42,43,44, and hence the fraction of ‘active’ TASN moieties, i.e., moieties dissociated in response to mechanical stress induced by crystallization would increase (Fig. 3g). (2) When comparing the topology between linear and star-shaped polymers that contain arms of identical molecular weight (Fig. 3f), S-PCL polymers have more arms involved in the formation of lamellar layers, which would lead to a further increase of ‘active’ TASN moieties that serve as tie molecules compared to L-PCL polymers (Fig. 3h). The mechano-activation behavior strongly depends on the environment of the mechanophore11. In solution under ultrasonication, single mechanophores at the center of the main chain are more likely to be activated in polymers with high molecular length45,46. On the other hand, in the bulk state under exposure to grinding, the mechano-responsiveness of mechanochromophores, especially radical-type mechanophores such as TASN, is governed by both the molecular weight and the number of branches47,48. The insights into mechano-responsiveness in polymer crystallization obtained in this paper are expected to broaden the understanding of polymer mechanochemistry.
Fluorescence microscopy measurements of CIMF
The visualization of the crystallization process, which is the most important part of this paper, was performed by confocal laser scanning microscopy (CLSM). So far, the twisting behavior of lamellar crystals in spherulites49,50,51,52 has been examined by polarized light microscopy38,53, small-angle light scattering54, transmission electron microscopy30, scanning electron microscopy55, and atomic force microscopy29,56,57. However, reports on the residual stress of the twisting behavior during the growth of spherulites remain elusive. Here, we addressed this dearth by converting the micro-mechanical forces during polymer crystallization into fluorescence arising from dissociated TASN radicals generated during the growth of the spherulites.
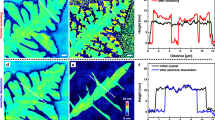
As shown in Fig. 4a (Supplementary Movie 1), microscopy images of L-PCL (Mn = 24,400) recorded at 30 °C reveal that spherulites grow from the molten state. Furthermore, a comparison with the bright-field images (Supplementary Fig. 22) demonstrated that the fluorescence emission is observed only in the growth direction of the lamellar crystals, while C1-PCL, which does not contain TASN moieties, exhibited no fluorescence (Fig. 4b). In contrast, C2-PCL, which contains a fluorescein moiety, exhibited fluorescence in both the crystalline and the amorphous regions (Supplementary Fig. 27). As shown in Fig. 4c, the fluorescence intensity increased during isothermal crystallization with increasing crystallinity, which was estimated based on DSC measurements under identical thermal conditions. The strong relationship between the fluorescence intensity and the crystallinity indicates that the stress generated during the growth of the spherulites can be evaluated at the molecular level. This tool could thus be used to evaluate micro-mechanical forces during polymer crystallization in the field of crystal growth, and also as a guideline for the molecular design of advanced materials.
a Microscopic images of L-PCL (Mn = 24,400) captured by confocal laser scanning microscopy (CLSM; λex = 514 nm; scale bar: 100 μm) during isothermal crystallization. b Microscopic images of C-PCL (Mn = 11,800) under bright-field conditions (left) and under excitation (right; λex = 514 nm; scale bar: 100 μm) during isothermal crystallization. c Normalized fluorescence intensity (blue open circles) and normalized crystallinity (red closed squares) during isothermal crystallization for L-PCL (Mn = 24,400). d 3D image of L-PCL (Mn = 24,400) alpha mode captured by CLSM (λex = 514 nm; scale bar: 100 μm) after isothermal crystallization.
We also confirmed that the distribution of the skewness and kurtosis in the fluorescence region (Supplementary Fig. 23) decreased with the growth of the lamellar crystals, which indicates that the spherulites are growing uniformly in two dimensions. CLSM also enables scanning the spherulites in 3D scan mode58 (Fig. 4d, Supplementary Figs. 25 and 26) as well as in airyscan mode (Supplementary Fig. 24), which allows detailed observations on the growth of lamellar crystals. It should be noted that the direct visualization of polymer crystallization with mechano-activated fluorescent radicals offers unprecedented insight into crystal growth processes.
Discussion
Radical-type mechanofluorophores such as tetraarylsuccinonitrile (TASN) allow us to evaluate the micro-mechanical forces during polymer crystallization in crystalline polymers by electron paramagnetic resonance (EPR) spectroscopy and to visualize it by fluorescence microscopy. The radical-generating behavior and the real-time stress visualization have revealed the relationship between crystalline polymer architecture and the efficiency of the force transmission during the growth of spherulites. The increase of molecular weight of the main chain affects the dissociation of TASN at tie molecules, whereby star-shaped polycaprolactone contain more micro-mechanical forces during polymer crystallization at tie molecules than linear polycaprolactones. This strategy can be expected to be applicable to a wide variety of polymers in order to evaluate their micro-mechanical forces during polymer crystallization, which may ultimately lead to design guidelines for advanced materials. We are convinced that this report on crystallization-induced mechanofluorescence (CIMF) will open research avenues in polymer and materials sciences.
Methods
Preparation of polymers containing TASN moiety
The synthesis, purification, and characterization, which includes the NMR, IR, DSC and GPC data, mechanical characterization, control experiments, and additional details for all polymers, are described in detail in Supplementary Information.
Evaluations of dissociated TASN radicals during polymer crystallization
Variable-temperature EPR measurements were carried out on a JEOL JES-X320 X-band EPR spectrometer equipped with a JEOL DVT temperature controller. The samples were filled to 5 mm glass capillaries, and the capillaries were sealed after being degassed. In case of isothermal crystallization, the glass capillaries were heated to 70 °C to melt the crystalline polymer absolutely, then the sample was kept at 30 °C (cooling rate: –20 °C min−1) and measured. The spectra of samples were measured using microwave power of 0.1 mW and a field modulation of 0.1 mT with a time constant of 0.03 s and a sweep rate of 0.25 mTs−1. The concentration of the radicals formed from the cleavage of TASN was determined by comparing the area of the observed integral spectrum with a 0.01 mM solution of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) in benzene under the same experimental conditions. The Mn2+ signal was used as an auxiliary standard.
CLSM experiments to observe crystallization-induced mechanofluorescence
The expression of mechano-activated fluorescence from TASN moiety at the center of the main chain was visualized by a Zeiss LSM880 with Airyscan (Carl Zeiss, Oberkochen, Germany) with ×20 objective lens using 514 nm excitation and 526–651 nm emission wavelengths for L-PCL, S-PCL, and C1-PCL. For isothermal crystallization, the samples placed on the cover glass (18 mm × 18 mm) were heated to 70 °C, then the sample was kept at 30 °C. As a control sample, C2-PCL was visualized using 488 nm excitation and 506–647 emission wavelengths. All samples were prepared by spin coater MSC-200D (Japan Create Co., Ltd.). The spin coating conditions are as following; polymer concentration: 120 mg/mL, rotational speed of disk: 800 rpm, 30 s, solvent: 1,2-dichloroethane.
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. All other data are available from the corresponding author upon reasonable request.
References
Zhang, Y., Ben Jar, P. Y., Xue, S. & Li, L. Quantification of strain-induced damage in semi-crystalline polymers: a review. J. Mater. Sci. 54, 62–82 (2018).
Bernhard, W. Macromolecular Physics. 1–3 (Academic Press, 1973).
Sanchez, L. A. & Hornberger, L. E. Monitoring of residual stresses in injection-molded plastics with holographic interferometry. Opt. Lasers Eng. 37, 27–37 (2002).
Maxwell, A. S. & Turnbull, A. Measurement of residual stress in engineering plastics using the hole-drilling technique. Polym. Test. 22, 231–233 (2003).
Pittman, J. F. T., Whitham, G. P., Beech, S. & Gwynn, D. Cooling and wall thickness uniformity in plastic pipe manufacture -an experimental study and computer simulations-. Int. Polym. Proc. 9, 130–140 (1994).
Seguela, R. Critical review of the molecular topology of semicrystalline polymers: the origin and assessment of intercrystalline tie molecules and chain entanglements. J. Polym. Sci. B Polym. Phys. 43, 1729–1748 (2005).
Huang, Y.-L. & Brown, N. Dependence of slow crack growth in polyethylene on butyl branch density: morphology and theory. J. Polym. Sci. B Polym. Phys. 29, 129–137 (1991).
Gautam, S., Balijepalli, S. & Rutledge, G. C. Molecular simulations of the interlamellar phase in polymers: effect of chain tilt. Macromolecules 33, 9136–9145 (2000).
Nilsson, F., Lan, X., Gkourmpis, T., Hedenqvist, M. S. & Gedde, U. W. Modelling tie chains and trapped entanglements in polyethylene. Polymer 53, 3594–3601 (2012).
Beyer, M. K. & Clausen-Schaumann, H. Mechanochemistry: the mechanical activation of covalent bonds. Chem. Rev. 105, 2921–2948 (2005).
Li, J., Nagamani, C. & Moore, J. S. Polymer mechanochemistry: from destructive to productive. Acc. Chem. Res. 48, 2181–2190 (2015).
Izak-Nau, E., Campagna, D., Baumann, C. & Göstl, R. Polymer mechanochemistry-enabled pericyclic reactions. Polym. Chem. 11, 2274–2299 (2020).
Min, Y. Q. et al. Sonochemical transformation of epoxy-amine thermoset into soluble and reusable polymers. Macromolecules 48, 316–322 (2015).
Piermattei, A., Karthikeyan, S. & Sijbesma, R. P. Activating catalysts with mechanical force. Nat. Chem. 1, 133–137 (2009).
Zhang, H. et al. Mechanochromism and mechanical-force-triggered cross-linking from a single reactive moiety incorporated into polymer chains. Angew. Chem. Int. Ed. 55, 3040–3044 (2016).
Chen, Z. et al. Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene. Science 357, 475–479 (2017).
Davis, D. A. et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 459, 68–72 (2009).
Chen, Y. et al. Mechanically induced chemiluminescence from polymers incorporating a 1,2-dioxetane unit in the main chain. Nat. Chem. 4, 559–562 (2012).
Robb, M. J. et al. Regioisomer-specific mechanochromism of naphthopyran in polymeric materials. J. Am. Chem. Soc. 138, 12328–12331 (2016).
Larsen, M. B. & Boydston, A. J. “Flex-activated” mechanophores: using polymer mechanochemistry to direct bond bending activation. J. Am. Chem. Soc. 135, 8189–8192 (2013).
Clough, J. M., van der Gucht, J. & Sijbesma, R. P. Mechanoluminescent imaging of osmotic stress-induced damage in a glassy polymer network. Macromolecules 50, 2043–2053 (2017).
Imato, K. et al. Mechanophores with a reversible radical system and freezing-induced mechanochemistry in polymer solutions and gels. Angew. Chem. Int. Ed. 54, 6168–6172 (2015).
Kato, S., Ishizuki, K., Aoki, D., Goseki, R. & Otsuka, H. Freezing-induced mechanoluminescence of polymer gels. ACS Macro Lett. 7, 1087–1091 (2018).
Kato, S., Aoki, D. & Otsuka, H. Introducing static cross-linking points into dynamic covalent polymer gels that display freezing-induced mechanofluorescence: enhanced force transmission efficiency and stability. Polym. Chem. 10, 2636–2640 (2019).
Verstraeten, F., Gostl, R. & Sijbesma, R. P. Stress-induced colouration and crosslinking of polymeric materials by mechanochemical formation of triphenylimidazolyl radicals. Chem. Commun. 52, 8608–8611 (2016).
Sumi, T., Goseki, R. & Otsuka, H. Tetraarylsuccinonitriles as mechanochromophores to generate highly stable luminescent carbon-centered radicals. Chem. Commun. 53, 11885–11888 (2017).
Crescenzi, V., Manzini, G., Calzolari, G. & Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 8, 449–463 (1972).
Wang, Y., Rodriguez-Perez, M. A., Reis, R. L. & Mano, J. F. Thermal and thermomechanical behaviour of polycaprolactone and starch/polycaprolactone blends for biomedical applications. Macromol. Mater. Eng. 290, 792–801 (2005).
Beekmans, L. G. M. & Vancso, G. J. Real-time crystallization study of poly(ε-caprolactone) by hot-stage atomic force microscopy. Polymer 41, 8975–8981 (2000).
Ikehara, T., Jinnai, H., Kaneko, T., Nishioka, H. & Nishi, T. Local lamellar structures in banded spherulites analyzed by three-dimensional electron tomography. J. Polym. Sci. B Polym. Phys. 45, 1122–1125 (2007).
Toda, A., Taguchi, K. & Kajioka, H. Growth of banded spherulites of poly(ε-caprolactone) from the blends: an examination of the modeling of spherulitic growth. Polymer 53, 1765–1771 (2012).
Wang, K., Cai, L., Jesse, S. & Wang, S. Poly(ε-caprolactone)-banded spherulites and interaction with MC3T3-E1 cells. Langmuir 28, 4382–4395 (2012).
Nojima, S., Ono, M. & Ashida, T. Crystallization of block copolymers II. morphological study of poly (ethylene glycol)-Poly (ε-caprolactone) block copolymers. Polym. J. 24, 1271–1280 (1992).
Nojima, S., Inokawa, D., Kawamura, T. & Nitta, K. Dynamic mechanical study of block copolymer crystallization confined within spherical nanodomains. Polym. J. 40, 986–991 (2008).
Kossack, W. & Kremer, F. Banded spherulites and twisting lamellae in poly–ε–caprolactone. Colloid Polym. Sci. 297, 771–779 (2019).
Makiguchi, K., Satoh, T. & Kakuchi, T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules 44, 1999–2005 (2011).
Saito, T., Aizawa, Y., Tajima, K., Isono, T. & Satoh, T. Organophosphate-catalyzed bulk ring-opening polymerization as an environmentally benign route leading to block copolyesters, end-functionalized polyesters, and polyester-based polyurethane. Polym. Chem. 6, 4374–4384 (2015).
Hoffman, J. D. & Miller, R. L. Test of the reptation concept: crystal growth rate as a function of molecular weight in polyethylene crystallized from the melt. Macromolecules 21, 3038–3051 (1988).
Abe, H., Kikkawa, Y., Inoue, Y. & Doi, Y. Morphological and kinetic analyses of regime transition for poly[(S)-lactide] crystal growth. Biomacromolecules 2, 1007–1014 (2001).
Tsuji, H., Tezuka, Y., Saha, S. K., Suzuki, M. & Itsuno, S. Spherulite growth of l-lactide copolymers: effects of tacticity and comonomers. Polymer 46, 4917–4927 (2005).
Jancar, J. & Fiore, K. Molecular weight scaling of the spherulite growth rate in isothermally melt crystallized polyethylene nanocomposites. Polymer 52, 5851–5857 (2011).
Phillips, P. J. & Rensch, G. J. Crystallization studies of poly(ɛ-caprolactone). II. Lamellar thickening melting.J. Polym. Sci. B Polym. Phys. 27, 155–173 (1989).
Adhikari, S. & Muthukumar, M. Theory of statistics of ties, loops, and tails in semicrystalline polymers. J. Chem. Phys. 151, 114905 (2019).
Zhai, Z., Morthomas, J., Fusco, C., Perez, M. & Lame, O. Crystallization and molecular topology of linear semicrystalline polymers: simulation of uni- and bimodal molecular weight distribution systems. Macromolecules 52, 4196–4208 (2019).
Church, D. C., Peterson, G. I. & Boydston, A. J. Comparison of mechanochemical chain scission rates for linear versus three-arm star polymers in strong acoustic fields. ACS Macro Lett. 3, 648–651 (2014).
May, P. A., Munaretto, N. F., Hamoy, M. B., Robb, M. J. & Moore, J. S. Is Molecular weight or degree of polymerization a better descriptor of ultrasound-induced mechanochemical transduction? ACS Macro Lett. 5, 177–180 (2016).
Oka, H. et al. Enhancing mechanochemical activation in the bulk state by designing polymer architectures. ACS Macro Lett. 5, 1124–1127 (2016).
Watabe, T., Ishizuki, K., Aoki, D. & Otsuka, H. Mechanochromic dendrimers: the relationship between primary structure and mechanochromic properties in the bulk. Chem. Commun. 55, 6831–6834 (2019).
Crist, B. & Schultz, J. M. Polymer spherulites: a critical review. Prog. Polym. Sci. 56, 1–63 (2016).
Shtukenberg, A. G., Punin, Y. O., Gujral, A. & Kahr, B. Growth actuated bending and twisting of single crystals. Angew. Chem. Int. Ed. 53, 672–699 (2014).
Lovinger, A. J. Twisted crystals and the origin of banding in spherulites of semicrystalline polymers. Macromolecules 53, 741–745 (2020).
Woo, E. M., Lugito, G. & Nagarajan, S. Dendritic polymer spherulites: birefringence correlating with lamellae assembly and origins of superimposed ring bands. J. Polym. Res. 27, 7 (2019).
Ye, L., Qiu, J., Wu, T., Shi, X. & Li, Y. Banded spherulite templated three-dimensional interpenetrated nanoporous materials. RSC Adv. 4, 43351–43356 (2014).
Baert, J. & Van Puyvelde, P. Density fluctuations during the early stages of polymer crystallization: an overview. Macromol. Mater. Eng. 293, 255–273 (2008).
Wang, Z. et al. Twisting of lamellar crystals in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) ring-banded spherulites. Macromolecules 43, 4441–4444 (2010).
Jiang, Y. et al. Lamellar branching of poly(bisphenol A-co-decane) spherulites at different temperatures studied by high-temperature AFM. Macromolecules 36, 3652–3655 (2003).
Mareau, V. H. & Prud’homme, R. E. In-situ hot stage atomic force microscopy study of poly(ε-caprolactone) crystal growth in ultrathin films. Macromolecules 38, 398–408 (2005).
Peng, H., Ruan, Z., Long, F., Simpson, J. H. & Myers, E. W. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets. Nat. Biotechnol. 28, 348–353 (2010).
Acknowledgements
This work was supported by KAKENHI grants 17H01205 (H.O.) from the Japan Society for the Promotion of Science (JSPS), JSPS Research Fellowships for Young Scientists 19J23009 (S.K.), JST CREST (grant number JPMJCR1991, H.O.), and JST ERATO (grant number JPMJER1602, K.N).
Author information
Authors and Affiliations
Contributions
S.K., S.F., D.A., R.G., and H.O. conceived the concept, designed the experiments. S.K. and S.F. performed the experiments and analyzed the data. S.K., S.F., D.A., R.G., K.O., K.T., N.S., A.M., K.N., and H.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kato, S., Furukawa, S., Aoki, D. et al. Crystallization-induced mechanofluorescence for visualization of polymer crystallization. Nat Commun 12, 126 (2021). https://doi.org/10.1038/s41467-020-20366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-20366-y
This article is cited by
-
Stimuli-responsive smart polymers based on functional dyes
Polymer Journal (2024)
-
Robust liquid crystal semi-interpenetrating polymer network with superior energy-dissipation performance
Nature Communications (2024)
-
Liquid-crystal elastomers based on covalent adaptable networks: From molecular design to applications
Science China Materials (2023)
-
The molecular mechanism of constructive remodeling of a mechanically-loaded polymer
Nature Communications (2022)
-
Bowls, vases and goblets—the microcrockery of polymer and nanocomposite morphology revealed by two-photon optical tomography
Nature Communications (2021)