Abstract
Magnetic refrigeration (MR) is a key technique for hydrogen liquefaction. Although the MR has ideally higher performance than the conventional gas compression technique around the hydrogen liquefaction temperature, the lack of MR materials with high magnetic entropy change in a wide temperature range required for the hydrogen liquefaction is a bottle-neck for practical applications of MR cooling systems. Here, we show a series of materials with a giant magnetocaloric effect (MCE) in magnetic entropy change (-∆Sm > 0.2 J cm−3K−1) in the Er(Ho)Co2-based compounds, suitable for operation in the full temperature range required for hydrogen liquefaction (20-77 K). We also demonstrate that the giant MCE becomes reversible, enabling sustainable use of the MR materials, by eliminating the magneto-structural phase transition that leads to deterioration of the MCE. This discovery can lead to the application of Er(Ho)Co2-based alloys for the hydrogen liquefaction using MR cooling technology for the future green fuel society.
Similar content being viewed by others
Introduction
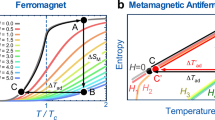
Magnetic materials undergo isothermal magnetic entropy changes (ΔSm) or adiabatic temperature changes (ΔTad) upon the application or removal of an external magnetic field. This phenomenon is known as the magnetocaloric effect (MCE)1. Magnetic refrigeration (MR) based on the MCE is considered to be a promising energy-efficient and environmentally benign refrigeration technology2. The concept of cooling by adiabatic demagnetisation at the ultra-low temperatures was proposed independently by Debye3 and Giauque4 based on thermodynamic studies in the late 1920s. This concept was experimentally demonstrated in the early 1930s by Giauque and MacDougall on the adiabatic demagnetisation of Gd2(SO4)3.8H2O, leading to attainment of temperatures below 1K5. The operation of MR can be also extended to room temperature application using magnetic refrigerants, such as Gd5Si2Ge26,7,8, (Mn,Fe)2P9, MnAs10,11, Ni-Mn-based Heusler alloy12,13 and La(Fe,Si)13H14,15,16. Recently, the global demand for the reduction of CO2 emission has increased the attention devoted to the use of renewable energy for which hydrogen plays an important role in the so-called decarbonised hydrogen society17. In this context, magnetic refrigeration has been demonstrated to be a potential candidate for hydrogen liquefaction and avoidance of hydrogen boil-off during storage18. In this approach, hydrogen gas is cooled to ~77 K (boiling temperature of liquid nitrogen) followed by a further decrease in temperature to its liquefaction temperature of 20 K. Because the existing magnetic refrigerant materials cannot maintain a large MCE over the wide temperature span of 77–20 K, a series of refrigerant materials are used in an active magnetic regenerator (AMR) system (Fig. 1a).
a Schematic of the active magnetic regenerator for hydrogen liquefaction. A series of magnetic refrigerants with tailored transition temperatures at external field (µ0Hext) are required to cover the large temperature range from the boiling temperature of nitrogen (77 K) to the boiling temperature of hydrogen (20 K). b Isothermal magnetic entropy change (−∆Sm) as a function of temperature. Second-order magnetic phase transition materials illustrated by dashed blue curves, for example, HoB220, HoNi224, HoAl228, and DyAl228 show no hysteresis, a giant MCE only at temperatures below 20 K and small MCE at temperatures above 30 K. First-order magnetic phase transition materials indicated by black curves with symbols exhibit giant magnetocaloric effects (MCEs) (large −∆Sm) at a broad temperature range but with thermal hysteresis near the transition temperature, resulting in irreversible giant MCEs. Therefore, giant and reversible MCEs with broad operating temperature window illustrated by gradient colour from red to blue corresponding to the magnetic refrigerants with different transition in (a) from 77 K to 20 K are desirable for active magnetic regenerator.
The isothermal magnetic entropy change (ΔSm) is given by
and is used to characterise the magnetocaloric response of a magnetic refrigerant, where µ0 is the permeability of free space, M is the magnetisation, T is the temperature, and H is the external magnetic field. The maximum value of ΔSm is achieved near the magnetic transition temperature (Ttr) owing to the large value of \(\frac{\partial M}{\partial T}\). HoAl2 compound is one of the potential magnetic refrigerant materials for hydrogen liquefaction, which has −△Sm = 0.16 J cm−3K−1at temperature of 32 K obtained from a single crystal and is called as a material with a giant MCE1,19. To realise an efficient AMR system for H2 liquefaction applications, a larger magnetic entropy change of −△Sm > 0.2 J cm−3K−1 covering a broad temperature range of 20–77 K for magnetic refrigerants is needed. Giant MCEs (−△Sm > 0.2J cm−3K−1) have been realised in Ho-based compounds (Fig. 1b), such as HoB220, HoN21,22, and HoNi223,24 at temperatures below 20 K. These ferro/paramagnetic phase transitions without thermal hysteresis have been classified as second-order magnetic phase transitions (SOPT). SOPT materials without thermal hysteresis intrinsically lead to reversible MCE and mechanical stability during cyclic performance25,26, and hence are desirable for practical application. To meet the requirements of AMR, giant MCE must be maintained at temperatures up to 77 K. However, the increase in Ttr of SOPT materials has been found to be achieved at the expense of |△Sm|. For example, |△Sm| values of 0.17 J cm−3K−1 and 0.15 J cm−3K−1 are obtained at 30 K for the (GdxHo1-x)B2 and HoAl2 compounds27,28, respectively, and |△Sm| decreases to 0.10 J cm−3K−1 at 60 K for the DyAl2 compound28. Hence, there are very few refrigerant materials exhibiting a giant MCE of |△Sm| > 0.2 J cm−3K−1 that do not show thermal hysteresis at the temperatures of 30–77 K required for H2 liquefaction in AMR systems. By contrast, magnetic refrigerant materials with first-order magnetic phase transition (FOPT) have giant MCEs induced by their magnetostructural phase transitions. For example, in ErCo2, a transformation from the paramagnetic phase (cubic) to the ferrimagnetic phase (rhombohedral) results in −△Sm = 0.37 J cm−3K−1 at 37 K (Fig. 1b)29. With Ho substitution for Er, Ttr can be increased to 80 K while maintaining a giant MCE (−△Sm > 0.2 J cm−3K−1) in the (Ho1-xErx)Co2 compounds30. However, the (Ho1-xErx)Co2 compounds undergoing FOPT suffer from thermal hysteresis resulting in an irreversible MCE and mechanical instabilities25,26, hindering their application for AMR.
In this work, we demonstrate that for (Ho)ErCo2 compounds, the substitution of Co by particular 3d metal elements, such as Fe or Fe + Ni, can eliminate the hysteresis by avoiding the structural transformation, while maintaining a giant MCE at the transition temperature. Different from the conventional reports, the narrow operating temperature window is substantially expanded in this work by developing a series of materials with hysteresis-free and tailored transitions at the temperature range of 20–77 K with |△Sm| > 0.2 J cm−3K−1. Thus, this discovery can directly lead to the realisation of the AMR system as a great leap toward the application of magnetic cooling technology for hydrogen liquefaction.
Results
Tuning the transition temperature and thermal hysteresis
Magnetisation as a function of temperature M(T) at a magnetic field of 1T is plotted for ErCo2 and Er(Co,Fe,Ni)2-based alloys (Fig. 2a, b). The ErCo2 alloy itself undergoes the paramagnetic/ferrimagnetic transition at TC = 37 K that accompanies a magnetostructural phase transition that gives rise to an abrupt change in the magnetisation, and correspondingly induces a giant magnetic entropy change of 0.37 J cm−3K−1 (supplementary Fig. 1). This comes at the cost of thermal hysteresis of 2 K (Fig. 2a), hindering the reversibility of MCE and the practical application of these materials. Figure 2a, b show that the partial substitution of Co with Fe or Fe + Ni solves this problem. The elimination of the thermal hysteresis was successfully achieved in the ErCo1.96Fe0.04 alloy with a broad transition temperature span, showing the features of SOPT. Further increase of Fe substitution for Co leads to less sharp transitions observed from the M-T curves and a reduction of magnetic entropy changes, as shown in supplementary Fig. 1. Tunable transition temperature is another requirement of magnetocaloric materials for AMR systems. As observed from Fig. 2a, the transition temperature increased from 37 K to 62 K when x increased to 0.07 in the ErCo2−xFex alloy while hysteresis was eliminated. The transition temperature can also be tuned toward a lower temperature (28 K) when Ni is alloyed in the ErCo1.96Fe0.04 alloy, while maintaining the hysteresis-free state, as shown in Fig. 2b. Refrigeration capacity (RC), defined as the integrated area under the -ΔSm(T) curve at the peak’s half value is another important parameter that is a measure for the amount of heat transfer from the cold to the hot reservoirs in a single ideal MR cycle. The RC is improved from 2.2 J cm−3 for ErCo2 to 2.42 J cm−3 in the case of the ErCo1.96Fe0.04 alloy. With a further increase in Fe content, a magnetic entropy change of 0.17 J cm−3K−1 at 57 K can be obtained for the ErCo1.95Fe0.05 alloy with an enhanced RC of 2.95 J cm−3. Although conventionally, a larger RC is achieved at the expense of the peak value of -ΔSm, making the use of magnetic refrigerants with SOPT less attractive, the |ΔSm| value for the ErCo2-xFex (x ≥ 0.04) alloys developed in this study induces the largest entropy change of 0.21 J cm−3K−1 within the temperature range of 30–77 K compared to all known refrigerant materials without hysteresis reported to date1,21,22,23,24,27,28,31,32, Arrott plots were employed (Fig. 2c, d) to determine the order of the magnetic phase transitions. A negative slope and reflection points near the transition temperature were observed for the ErCo2 alloy, revealing a typical FOPT based on the Banerjee criterion33. The negative slope was strongly suppressed, and a characteristic of SOPT was observed for the compositionally-modified ErCo1.95Fe0.05 alloy.
a, b The Magnetisation (M) is plotted versus temperature at an applied magnetic field of 1T showing that the transition temperature can be tuned to higher temperatures (a) and to lower temperatures (b), while eliminating the hysteresis by substituting Co by Fe and Fe + Ni in the ErCo2-based system. The colours of the curves in a, b correspond to the colour of listed alloy compositions. c, d The Arrott plots measured from 27 K to 69 K at a temperature step (△T) of 3 K for c ErCo2 and d ErCo1.95Fe0.05 alloy show the changes of the phase transition from first-order to second-order, respectively.
Characterisation of phase transitions
Although the Arrott plots qualitatively show the nature of the magnetic phase transition, the latter can be investigated further by tracking the change in the crystal lattice during the phase transition. An unambiguous assessment of the phase transition was carried out using in-situ XRD measurement under cryogenic temperatures. The details of the determination of the phase transition by XRD are shown in the supplementary note 1. The change in the lattice spacing upon cooling from 100 to 5 K is plotted in Fig. 3a. Because the quantities \(\sqrt{2}{a}_{r}\) and \({c}_{r}/\sqrt{3}\) of the lattice parameters for the rhombohedral phase are equivalent to the ac of the cubic lattice,\(\,\sqrt{2}{a}_{r}\) and \({c}_{r}/\sqrt{3}\) for the rhombohedral phase are plotted to compare directly with the lattice parameter of the cubic phase. A step jump in the lattice spacing in ErCo2 was observed near the transition temperature for the ErCo2 alloy. With Fe substitution for Co in the ErCo1.96Fe0.04 alloy, the lattice constant change was substantially reduced, even though a cubic/rhombohedral crystal structure change was observed. This result clearly shows that the addition of Fe can suppress the large volume change that occurs in the magnetostructural phase transition in ErCo2 at Ttr. A further increase in Fe content in the ErCo1.95Fe0.05 alloy leads to no changes in the lattice spacing or volume per chemical formula caused by the structural transition near Ttr, indicating the realisation of SOPT. In other words, this work demonstrates a concept that the substitution of a 3d metal element, such as Fe for Co in the ErCo2-based Laves phase eliminates the structural phase transition, thereby reducing the thermal hysteresis to 0 K.
a Temperature dependence of the lattice parameters for ErCo2-based alloys. The lattice parameters are obtained from the Rietveld refinement. The lattice parameters of the rhombohedral phase are plotted for \(\sqrt{2}{a}_{r}\) (black) and \({c}_{r}/\sqrt{3}\) (red) to compare directly with the lattice parameter of the cubic phase. b Temperature-dependent volume per chemical formula for ErCo2 (black), ErCo1.96Fe0.04 (purple) and ErCo1.95Fe0.05 (red) alloys, the crystal structure change upon transition is also illustrated by a unit cell (red and brown spheres represent Er and Co atoms, respectively, and red and brown arrows represent the magnetic moments of the Er and Co atoms, respectively).
A further characterisation of the nature of the phase transitions in Er(Co1-xFex)2 was performed by measuring the specific heat, \(C\), using the thermal relaxation method, the details of which are described in the supplementary note 2. In Fig. 4a, the specific heat values given by three measurements are the same in each temperature for the three samples (ErCo2, ErCo1.96Fe0.04 and ErCo1.95Fe0.05 alloys), within the experimental accuracy, apart from the temperatures close to the phase transition temperatures. Here, we analysed the dependence of the time evolution of the sample temperature on the phase transition temperatures for the three samples shown in Fig. 4b. For ErCo2, a significant difference in T(t) was found at the phase transition temperature of ~35 K, as indicated by arrow (I) in Fig. 4a. The temperature rise during the first run is significantly suppressed by the existing latent heat, compared to those in the second and third runs. The strong thermal hysteretic behaviour observed in Fig. 2 for the phase transition of ErCo2 corresponds to the FOPT behaviour. For sample II (ErCo1.96Fe0.04), a similar behaviour was observed (Fig. 4b), indicating that the phase transition is still first-order. It is clear that the \(\Delta {T}_{{\max }}\) value in the first measurement is lower than those in the second and third measurements. On the other hand, the hysteretic behaviour was not observed within the experimental accuracy in sample III (ErCo1.95Fe0.05) as shown in Fig. 4b. Thus, we conclude that the phase transitions for ErCo2 and ErCo1.96Fe0.04 are FOPT, while the ErCo1.95Fe0.05 sample does not show FOPT behaviour, which is consistent with that observed by in-situ XRD shown in Fig. 3.
a Temperature dependence of the specific heat measured by the thermal relaxation method for ErCo2 (black), ErCo1.96Fe0.04 (blue) and ErCo1.95Fe0.05 (red) alloys. b Time evolution of the sample temperature obtained using the relaxation method at the temperature where each sample shows a peak anomaly indicated by arrows (I), (II), and (III) in a. The dashed lines denote heat power for each measurement (1st (black), 2nd (blue) and 3rd (red) measurements) in b.
Origin of large magnetocaloric effect
Figure 5a shows the results of the specific magnetometric measurements of Er and Co probed by X-ray magnetic circular dichroism (XMCD). Note that the data for the ErCo2 alloy were adopted from reference34. The Co magnetisation at low temperatures (<10 K) remains opposite to the Er magnetisation after alloying with Fe, thus revealing that the ferrimagnetic behaviour is not changed by the substitution of Co with Fe. Unlike for ErCo2 alloy that exhibits a sharp change in the magnetic moment of Er at Ttr, the change in the magnetic moment of Er is more gradual around Ttr, which is consistent with the M–T curves presented in Fig. 2. The evolution of the magnetic moments of Er and Co can be directly seen from the XMCD signal shown in the Supplementary Fig. 2. In the XMCD signal of the Co L2,3 edges, an inversion of the edges appears at the temperatures above 56 K, corresponding to the change in the sign of the magnetic moment of Co. For the Er M4,5 XMCD spectra, the intensity of the signal decreases as the temperature increases. In-situ Lorentz microscopy observation in the Fresnel mode was employed to understand the evolution of magnetic domains during cooling. Figure 5b shows the selected micrographs in the cooling process at four different temperatures. These are also marked as i–iv and I–IV in Fig. 5a for ErCo2 and ErCo1.95Fe0.05 alloys, respectively. For both samples, there is no contrast in the paramagnetic state. The XMCD result in Fig. 5a shows that the magnetic moment of Er atoms increases upon cooling of the ErCo2 sample via the magnetic momentum alignment, while the net magnetic moment of Co remains almost zero. This results in the formation of fine and maze-like magnetic domain structures, as shown in ii in Fig. 5b. Note that close to the Ttr of ErCo2, a mountain-like contrast marked by red circles appears originating from a large strain caused by the cubic/rhombohedral structural transformation. The change in the magnetic domain structure upon cooling to 37 K is due to a substantial increase in the magnetic moment of Er by formation of ferrimagnetic phase, as shown in Fig. 5a. Note that unlike the ErCo2 sample, no distinct strain contours were observed in the ErCo1.95Fe0.05 sample because the cubic structure was preserved at the temperatures below 52 K in the latter case. The observed transition in the shape of the magnetic domains from II to IV, i. e. fine maze-like domain patterns in II, strip-like domain patterns in IV, and their mixture in III (indicated by black arrow heads), are due to the gradual increase in the magnetic moment of Er upon a decrease in temperature without existence of any strain contour. Note that the ErCo1.95Fe0.05 phase is in its ferrimagnetic state at the temperature of 10 K.
a Temperature-dependent magnetic moments for Er (red) and Co (blue) in ErCo234, and ErCo1.95Fe0.05 alloys. b Magnetic domain evolution in ErCo2-based alloys examined using cryogenic Lorentz microscopy in the Fresnel mode. The black arrow heads show the magnetic domains and red circles illustrate the mountain-like contrasts caused by strain; the crystal structure change upon transition is also shown by a unit cell (red and brown spheres for Er and Co atoms, respectively, and red and brown arrows for the magnetic moments of Er and Co, respectively).
The giant MCE in the ErCo2 alloy originates from the magnetostructural phase transition and itinerant electron metamagnetism (IEM). The latter was proposed by Wohlfarth and Rhodes35. According to the proposed concept, the creation of a magnetic moment in Co is a consequence of the IEM induced by localised ferromagnetic ordering of Er moments that induces a large exchange field at the transition temperature. This can be observed in Fig. 5a, wherein Co has nearly zero magnetisation in the paramagnetic state, while it antiferromagnetically couples with Er at temperatures below 37 K. In this study, we found SOPT with the substitution of Fe for Co in the ErCo1.95Fe0.05 alloy, in which no structural transformation was observed, while a giant MCE was induced. However, based on the XMCD results, the magnetic moment of Co at temperatures below Ttr remains comparable in ErCo2 and ErCo1.95Fe0.05 alloys, suggesting that IEM is preserved upon a small substitution of Fe for Co. In addition to the structural transition, any change in the density of states at the Fermi level between these two alloys influences the IEM in Fe-doped alloys36,37. We calculated the density of states using density functional theory (DFT) using experimental lattice constants (Supplementary Fig. 3). Trace amounts of Fe substitution for Co have negligible influence on the electronic structure around the Fermi level compared with the cubic/rhombohedral ErCo2. This implies that the instability of the 3d sublattice magnetism and IEM is preserved for the ErCo1.95Fe0.05 alloy, which is consistent with the experimental data obtained by XMCD (Fig. 5a). This observation is in contrast to the conventional belief that FOPT results from IEM36,37. Here, we demonstrate that by eliminating the structural phase transition, thermal hysteresis can be eliminated, while the giant MCE can still be achieved due to the preserved IEM in the ErCo1.95Fe0.05 alloy.
Discussion
In practical applications, the surface/volume ratio of the magnetic refrigerants should be increased to achieve better heat exchange between the refrigerants and heat-exchanger fluid. Hence, spherical particles are desired. We employed a gas atomisation method and obtained spherical particles with an average particle diameter in the range of 210–350 μm. An example of spherical particles of the ErCo1.96Fe0.04 alloy is shown in the inset of Fig. 6a. The optimal annealing of the gas-atomised powders (supplementary Fig. 4) led to the realisation of a −ΔSm value comparable to that of the bulk sample (Fig. 6a). To develop magnetic refrigeration materials suitable for a broad temperature range of 77–20 K, we further investigated different 3d metal dopants to ErCo2 and HoCo2-based compounds. We substituted Ni, Al, Fe, and their combinations for Co in Er(Ho)Co2 alloys. Figure 6b shows that hysteresis can be eliminated in conventional FOPT-type ErCo2 and HoCo2 alloys by the doping, and the transition temperature can be tuned from 20 K to 77 K. These changes enable the values of −ΔSm > 0.2 J cm−3K−1 to be achieved in the range from 20 to 77 K to cover the required temperature range for hydrogen liquefaction (Fig. 6c). The magnetic refrigerants developed in this study yielded significantly larger entropy changes than those of HoNi224, HoAl228, REMn2X238,39,40, RECo2Mnx41 and DyAl228 for cryogenic applications, particularly in the temperature range from 30 K to 77 K. For example, the −ΔSm values for the ErCo1.85Ni0.11Fe0.04 and HoCo1.8Ni0.15Al0.05 alloys are 55% and 93 % larger than those of HoAl2 and DyAl2 at the same transition temperatures. Further characterisation of ΔTad of magnetic refrigerants is depicted in Fig. 6d; above 4 K, ΔTad can be achieved for the magnetic refrigerants developed in this work that can cover the application temperature range from 20 to 77 K for hydrogen liquefaction.
a Comparison of the temperature-dependent magnetic entropy change (−ΔSm(T)) of a bulk sample (black) and gas-atomised (blue) ErCo1.96Fe0.04 particle. The inset shows a scanning electron microscopy (SEM) image of a gas-atomised sample. b Temperature-dependent magnetisation M(T) measurements of the cooling and heating branches from 5 K to 100 K at 1 K intervals (µ0H = 1T) for a series of magnetic refrigerants with tailored transition temperatures, the spherical particles in AMR with different colours correspond to different magnetic refrigerants with different transition temperatures in b. c Temperature-dependent magnetic entropy change −ΔSm(T) curves of magnetic refrigerants with tailored transition temperatures covering the large temperature window from 20 K to 77 K. d Adiabatic temperature change (ΔTad) at the field change (ΔH) of 5T of the selected magnetic refrigerants developed in this work at their transition temperatures. The selected colours of the curves in b–d correspond to the colour of listed alloy compositions.
Hysteresis is a widespread phenomenon resulting from the nature of the first-order phase transition in magnetic refrigerants that leads to the irreversibility of the magnetocaloric response. Herein, we demonstrate that the elimination of hysteresis can be realised by avoiding the crystal structure change across the phase transition, specifically by alloying with 3d elements, while maintaining the giant magnetocaloric effect comparable to that of a conventional FOPT Er(Ho)Co2 alloy. The role of Fe in suppressing the structural transformation upon phase transition is due to the change from FOPT to SOPT as revealed by in-situ XRD, latent heat measurement, and Lorentz TEM for ErCo1.95Fe0.05. Moreover, the giant MCE was maintained for SOPT because of the preservation of IEM as found by XMCD experiments and DFT calculations. The Er(Ho)Co2-based alloys developed in this study have numerous merits, such as significantly larger magnetic entropy changes compared with those of known materials in the temperature range of 20–77 K, a wide operating temperature window due to the stacking of the refrigerants covering the range of application temperatures for hydrogen liquefaction, easy fabrication, and stable magnetocaloric properties of gas-atomised particles. This may lead to the application of Er(Ho)Co2-based alloys for hydrogen liquefaction using an active magnetic regenerator. Moreover, the approach for achieving giant reversible MCE in this study is also expected to be applicable for eliminating the detrimental hysteresis of magnetic refrigerants with FOPT for applications near room temperature, such as Gd5Si2Ge26,7,8, (MnFe)2P9 and La(FeSi)13H14,15.
Methods
Sample preparation
Polycrystalline (Ho)ErCo2-based samples were prepared by arc melting pure constituent elements in an Ar atmosphere, with 2–5 wt.% extra Er and Ho added to compensate the evaporation during sample preparation. The ingots were remelted four times after flipping over to achieve better homogeneity. Thereafter, the ingots were sealed in quartz in an argon atmosphere and annealed for 50 h at 1273 K. The phase constituents and crystal structure were examined by XRD (Rigaku SmartLab 9 kW) with Cu Kα1 radiation in the temperature range of 5–300 K. Thermomagnetic measurements were conducted using a superconducting quantum interference device magnetometer (SQUID-VSM).
Thermomagnetic measurements
To directly measure the adiabatic temperature change, a zirconium oxynitride thin-film CernoxTM thermometer (CX-SD, Lake Shore Cryotronics) was placed on the large surface of a cubic-shaped sample and fixed by thin copper wires. The sample assembly was inserted into the quantum design physical property measurement system (PPMS). The temperature and magnetic field were controlled by PPMS. The sample space was pumped by a cryopump, and the pressure was maintained below 10−4 Torr to reach adiabatic conditions. The thermal relaxation method for latent heat measurements was implemented in the physical properties measurement system (PPMS) manufactured by Quantum Design. To evaluate the time evolution of sample temperature during measurement, the raw data were extracted for each measurement.
Cryogenic microstructure characterisations
Cryogenic Lorentz microscopy was conducted using ultra-high-voltage (1.2 MV) Hitachi-TEM instrument. The temperature of the TEM specimen was reduced to ~10 K in a cryostat TEM sample holder using liquid helium. The specimens for the TEM analysis were prepared by an FEI Helios G4-UX dual-beam system using the lift-out method. The soft XMCD spectra at the Er M4,5 and Co L2,3 edges were recorded using the total electron yield method at the BL25SU beamline of SPring-8. The XMCD signal (µm) was obtained as \({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{m}}}}}}}=({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{l}}}}}}-}+{{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{r}}}}}}+})-({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{l}}}}}}+}+{{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{r}}}}}}-})\), where \({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{r}}}}}}}\) and \({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{l}}}}}}}\) represent the X-ray absorption spectrum (XAS) for the helicity plus (\({{{{{{\rm{h}}}}}}}_{+}\)) and minus (\({{{{{{\rm{h}}}}}}}_{-}\)) of soft X-rays, respectively, and \({{{{{{\rm{\mu }}}}}}}_{+}\) and \({{{{{{\rm{\mu }}}}}}}_{-}\) respectively represent XAS for a positive and negative applied field with intensity equal to 1.9 T. The degree of circular polarisation was previously estimated as 0.96 at 400 eV42 and was expected to be similar or larger in the energy region used in the present work. The angle between the magnetic field and the incident X-ray beam was 10°(ref. 43). The rod-shaped sample was fractured in the ultra-high vacuum chamber of the XMCD apparatus with the vacuum level of <5 × 10−7 Pa to obtain a fresh surface for the XMCD experiments. The samples were measured at different temperatures between 10 and 100 K. The magnetic moments were calculated using the magneto-optical sum rule analysis for XMCD44,45,46 by taking into consideration the spin-correction factor necessary to apply the sum rules for rare earths47.
First-principles calculations
Density functional theory (DFT) calculations were performed using the full-potential linearised augmented plane wave (FLAPW) method as implemented in the WIEN2K code48. The exchange-correlation interaction was approximated using the Perdew–Burke–Ernzerhof (PBE)49 formulation based on the generalised gradient approximation (GGA). The k-point sampling of the Brillouin zone was performed using 11 × 11 × 11 k-mesh grids. The spin-orbit coupling (SOC) was included in the calculations self-consistently. The localised 4 f electrons of Er were treated based on the implementation of the Hubbard’s U parameter using the DFT + U method with U = 10 eV and J = 0.75 eV, including the orbital polarisation. The experimental lattice constants for the rhombohedral and cubic structures were used, and Fe doping in ErCo1.95Fe0.05 was simulated using the virtual crystal approximation50. The ferrimagnetic behaviour and 6 μB orbital moments for Er were confirmed in all systems.
Data availability
All data are available within the Article and Supplementary Files, or available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Gschneidner, K. A. Jr, Pecharsky, V. K. & Tsokol, A. O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 68, 1479–1539 (2005).
Franco, V. et al. Magnetocaloric effect: from materials research to refrigeration devices. Prog. Mater. Sci. 93, 112–232 (2018).
Debye, P. Einige bemerkungen zur magnetisierung bei tiefer temperatur. Ann. Phys. 386, 1154–1160 (1926).
Giauque, W. F. A thermodynamic treatment of certain magnetic effects. A proposed method of producing temperatures considerably below 1◦ absolute. J. Am. Chem. Soc. 49, 1864–1870 (1927).
Giauque, W. F. & MacDougall, D. P. 1933 Attainment of temperatures below 1◦ absolute by demagnetization of Gd2(SO4)3·8H2O. Phys. Rev. 43, 768 (1933).
Pecharsky, V. K. & Gschneidner, K. A. Jr Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 78, 4494–4497 (1997).
Provenzano, V., Shapiro, A. J. & Shull, R. D. Reduction of hysteresis losses in the magnetic refrigerant Gd5Ge2Si2 by the addition of iron. Nature 429, 853–857 (2004).
Moore, J. D. et al. Metamagnetism seeded by nanostructural features of single-crystalline Gd5Si2Ge2. Adv. Mater. 21, 3780–3783 (2009).
Tegus, O., Brück, E., Buschow, K. H. J. & de Boer, F. R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 415, 150–152 (2002).
Wada, H. & Tanabe, Y. Giant magnetocaloric effect of MnAs1− xSbx. Appl. Phys. Lett. 79, 3302–3304 (2001).
de Campos, A. et al. Ambient pressure colossal magnetocaloric effect tuned by composition in Mn1−xFexAs. Nat. Mater. 5, 802–804 (2006).
Krenke, T. et al. Inverse magnetocaloric effect in ferromagnetic Ni–Mn–Sn alloys. Nat. Mater. 4, 450–454 (2005).
Liu, J., Gottschall, T., Skokov, K. P., Moore, J. D. & Gutfleisch, O. Giant magnetocaloric effect driven by structural transitions. Nat. Mater. 11, 620–626 (2012).
Hu, F. X., Shen, B. G., Sun, J. R. & Cheng, Z. H. Influence of negative lattice expansion and metamagnetic transition on magnetic entropy change in the compound LaFe11.4Si1.6. Appl. Phys. Lett. 78, 3675–3677 (2001).
Fujita, A., Fujieda, S., Hasegawa, Y. & Fukamichi, K. Itinerant-electron metamagnetic transition and large magnetocaloric effects in La(FexSi1−x)13 compounds and their hydrides. Phys. Rev. B 67, 104416 (2003).
Lyubina, J., Nenkov, K., Schultz, L. & Gutfleisch, O. Multiple metamagnetic transitions in the magnetic refrigerant La(Fe,Si)13Hx. Phys. Rev. Lett. 101, 177203 (2008).
Jones, L. W. Liquid hydrogen as a fuel for the future. Science 174, 367–370 (1971).
Numazawa, T., Kamiya, K., Utaki, T. & Matsumoto, K. Magnetic refrigerator for hydrogen liquefaction. Cryogenics 62, 185–192 (2014).
Monteiro, J. C. B. & Gandra, F. G. Direct measurements of conventional and anisotropic magnetocaloric effect in binary RAl2 single crystals. J. Appl. Phys. 121, 213904 (2017).
Baptista de Castro, P. et al. Machine-learning-guided discovery of the gigantic magnetocaloric effect in HoB2 near the hydrogen liquefaction temperature. NPG Asia Mater. 12, 35 (2020).
Yamamoto, T. A., Nakagawa, T., Sako, K., Arakawa, T. & Nitani, H. Magnetocaloric effect of rare earth mono-nitrides, TbN and HoN. J. Alloys Compd 376, 17–22 (2004).
Hirayama, Y. et al. Magnetocaloric effect, specific heat and adiabatic temperature change of HoxEr1−xN (x = 0.25, 0.5, 0.75). J. Alloys Compd 462, L12–L15 (2008). pp.
von Ranke, P. J., Grangeia, D. F., Caldas, A. & de Oliveira, N. A. Investigations on magnetic refrigeration: application to RNi2 (R = Nd, Gd, Tb, Dy, Ho, and Er). J. Appl. Phys. 93, 4055–4059 (2003).
Lai, J., Tang, X., Sepehri-Amin, H. & Hono, K. Tuning magnetocaloric effect of Ho1-xGdxNi2 and HoNi2-yCoy alloys around hydrogen liquefaction temperature. Scr. Mater. 188, 302–306 (2020).
Gutfleisch, O. et al. Mastering hysteresis in magnetocaloric materials. Philos. Trans. R. Soc. A 374, 20150308 (2016).
Guillou, F., Porcari, G., Yibole, H., von Dijk, N. & Bruck, E. Taming the first-order transition in giant magnetocaloric materials. Adv. Mater. 26, 2671–2675 (2014).
Baptista de Castro, P. et al. Enhancement of giant refrigerant capacity in Ho1-xGdxB2 alloys (0.1 ≤ x ≤ 0.4). J. Alloys Compd 865, 158881 (2021).
Campoy, J. C. P., Plaza, E. J. R., Coelho, A. A. & Gama, S. Magnetoresistivity as a probe to the field-induced change of magnetic entropy in RAl2 compounds (R=Pr,Nd,Tb,Dy,Ho,Er). Phys. Rev. B 74, 134410 (2006).
Wada, H., Tomekawa, S. & Shiga, M. Magnetocaloric properties of a first-order magnetic transition system ErCo2. Cryogenics 39, 915–919 (1999).
Zhu, Y. et al. Magnetocaloric effect of (ErxR1-x)Co2 (R = Ho, Dy) for magnetic refrigeration between 20 and 80 K. Cryogenics 51, 494–498 (2011).
Zhang, X. X., Wang, F. W. & Wen, G. H. Magnetic entropy change in RCoAl (R = Gd, Tb, Dy, and Ho) compounds: candidate materials for providing magnetic refrigeration in the temperature range 10 K to 100 K. J. Phys. Condens. Matter 13, L747 (2001).
Das, P. K., Bhattacharyya, A., Kulkarni, R., Dhar, S. K. & Thamizhavel, A. Anisotropic magnetic properties and giant magnetocaloric effect of single-crystal PrSi. Phys. Rev. B 89, 134418 (2014).
Banerjee, B. K. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 12, 16–17 (1964).
Herrero-Albillos, J. et al. Observation of a different magnetic disorder in ErCo2. Phys. Rev. B 76, 094409 (2007).
Wohlfarth, E. P. & Rhodes, P. Collective electron metamagnetism. Philos. Mag. 7, 1817–1824 (1962).
Syshchenko, O., Fujita, T., Sechovský, V., Diviš, M. & Fujii, H. Magnetism in ErCo2 under high pressure. Phys. Rev. B 63, 054433 (2001).
Singh, N. K. et al. Itinerant electron metamagnetism and magnetocaloric effect in RCo2-based Laves phase compounds. J. Magn. Magn. 317, 68–79 (2007).
Wang, J. L. et al. Magnetocaloric effect in layered NdMn2Ge0.4Si1.6. Appl. Phys. Lett. 98, 232509 (2011).
Li, L. W. et al. Giant reversible magnetocaloric effect in ErMn2Si2 compound with a second order magnetic phase transition. Appl. Phys. Lett. 100, 152403 (2012).
Wang, J. L. et al. Driving magnetostructural transitions in layered intermetallic compounds. Phys. Rev. Lett. 110, 217211 (2013).
Gerasimov, E. G. et al. Structure, magnetic and magnetothermal properties of the non-stoichiometric ErCo2Mnx alloys. Intermetallics 140, 107386 (2022).
Hirono, T., Kimura, H., Muro, T., Saitoh, Y. & Ishikawa, T. Full polarization measurement of SR emitted from twin helical undulators with use of Sc/Cr multilayers at near 400 eV. J. Electron Spectrosc. Relat. Phenom. 144-147, 1097–1099 (2005).
Nakamura, T. et al. Development of a soft X-ray magnetic circular dichroism spectrometer using a 1.9 T electromagnet at BL25SU of SPring-8. J. Electron Spectrosc. Relat. Phenom. 144-147, 1035–1038 (2005).
Thole, B. T., Carra, P., Sette, F. & Van Der Laan, G. X-ray circular dichroism as a probe of orbital magnetization. Phys. Rev. Lett. 68, 1943–1946 (1992).
Carra, P., Thole, B. T., Altarelli, M. & Wang, X. X-ray circular dichroism and local magnetic fields. Phys. Rev. Lett. 70, 694–697 (1993).
Chen, C. T. et al. Experimental confirmation of the x-ray magnetic circular dichroism sum rules for iron and cobalt. Phys. Rev. Lett. 75, 152–155 (1995).
Teramura, Y., Tanaka, A., Thole, B. T. & Jo, T. Effect of Coulomb interaction on the X-ray magnetic circular dichroism spin sum rule in rare earths. J. Phys. Soc. Jpn 65, 3056–3059 (1996).
Schwarz, K., Blaha, P. & Madsen, G. K. H. Electronic structure calculations of solids using the WIEN2k package for material sciences. Comput. Phys. Commun. 147, 71–76 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Bellaiche, L. & Vanderbilt, D. Virtual crystal approximation revisited: Application to dielectric and piezoelectric properties of perovskites. Phys. Rev. B. 61, 7877–7882 (2000).
Acknowledgements
This work was supported by the JST-Mirai Program Grant Number JPMJMI18A3 and KAKENHI 19H05819, Japan. The synchrotron radiation experiments were performed at BL25SU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2020A1414 and 2020A2143). Cryogenic Lorentz microscopy was performed using research equipment shared in MEXT Project for promoting public utilisation of advanced research infrastructure (Programme for supporting introduction of the new sharing system), Grant Number JPMXS0410500020. H. Sepehri-Amin and T. Nakamura acknowledge support from NIMS-TOHOKU joint research partnership program. Xin Tang acknowledges the provision of ICYS fellowship.
Author information
Authors and Affiliations
Contributions
X.T. and H.S.A. raised the idea and designed the experiments. X. T. conducted the alloy developments, performed room temperature XRD and magneto-thermal measurement. H.S.A. led the project. H.S.A. and T.O. conducted TEM measurements. N.T. carried out ΔTad and specific heat measurements. A.M.C., S.K., Y.K. and T.N. performed XMCD measurements and analysed XMCD results. I.K. and Y.Mi. conducted theoretical DFT calculations. H.T. prepared the spherical particles of magnetic refrigerants using gas atomisation process. J.L. contributed in the thermomagnetic measurements and XRD analysis. Y.Ma. carried out in-situ XRD measurements and analysed the data. X.T., H.S.A., N.T. and K.H. interpreted the key findings and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jianli Wang and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, X., Sepehri-Amin, H., Terada, N. et al. Magnetic refrigeration material operating at a full temperature range required for hydrogen liquefaction. Nat Commun 13, 1817 (2022). https://doi.org/10.1038/s41467-022-29340-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-29340-2
This article is cited by
-
Highly reversible magnetocaloric effect in Gd5Si0.25Ge3.75 and Gd5Si0.5Ge3.5 under moderate magnetic fields for hydrogen liquefaction
Communications Materials (2025)
-
Accelerating commercial deployment with hydrogen system testbeds
Nature Energy (2025)
-
Elasticity and stability of GdAl2 under pressure and temperature investigated using DFT+AI
Scientific Reports (2025)
-
All-temperature barocaloric effects at pressure-induced phase transitions
Nature Communications (2025)
-
Thermodynamic analysis of magnetocaloric properties of ferromagnet undergoing structural phase transition near Curie temperature
Scientific Reports (2025)