Abstract
Photocatalytic coupling of methane to ethane and ethylene (C2 compounds) offers a promising approach to utilizing the abundant methane resource. However, the state-of-the-art photocatalysts usually suffer from very limited C2 formation rates. Here, we report our discovery that the anatase TiO2 nanocrystals mainly exposing {101} facets, which are generally considered less active in photocatalysis, demonstrate surprisingly better performances than those exposing the high-energy {001} facet. The palladium co-catalyst plays a pivotal role and the Pd2+ site on co-catalyst accounts for the selective C2 formation. We unveil that the anatase {101} facet favors the formation of hydroxyl radicals in aqueous phase near the surface, where they activate methane molecules into methyl radicals, and the Pd2+ site participates in facilitating the adsorption and coupling of methyl radicals. This work provides a strategy to design efficient nanocatalysts for selective photocatalytic methane coupling by reaction-space separation to optimize heterogeneous-homogeneous reactions at solid-liquid interfaces.
Similar content being viewed by others
Introduction
As the major constituent of natural gas, shale gas, biogas, and combustible ice, methane has attracted much attention for the production of value-added chemicals and easily transportable fuels. The current industrial process for the chemical utilization of methane is limited to reforming to offer syngas (CO + H2), followed by syngas transformations. As compared to this indirect route, a direct methane conversion route would be more energy- and cost-efficient, and the development of the direct methane conversion route has long been regarded as one of the most fascinating research goals1. Among various possible methane valorization reactions, the non-oxidative coupling of methane (NOCM) to C2 compounds (ethane and ethylene) is of particular interest because of the simultaneous production of valuable H2 and the potential zero emission of CO22,3. However, the NOCM to either ethane or ethylene is a highly endothermic reaction and is thermodynamically limited under mild conditions. A high reaction temperature (> 650 °C) is required to trigger the NOCM by thermocatalysis, usually leading to deep dehydrogenative by-products or even carbon deposition4.
Photocatalysis, driven by solar energy, provides a promising way for the transformation of methane to high-value chemicals under mild conditions5,6,7,8,9,10,11,12,13. Many recent studies have been devoted to developing the NOCM photocatalysts14, and metal oxide (e.g., TiO2, ZnO, and TiO2 − SiO2)-based semiconductors loaded or doped with various co-catalysts have shown promising performances for the formation of C2 compounds15,16,17,18,19,20,21. It has been proposed that the photogenerated hole centers on oxide surfaces (O2− + h+ \(\longrightarrow\) O•−) or hydroxyl radicals (•OH) in the presence of H2O may be responsible for activating the C−H bond in CH4 under mild conditions, forming •CH3 for C−C coupling to C2H6 as the major product13. However, these active oxygen species with strong oxidation ability are also known to cause uncontrollable oxidation reactions12,13,22, lowering the formation rate of C2 compounds12,13,14. As a result, the C2 formation rate can hardly exceed 100 µmol g−1 h−1 over most of the photocatalysts reported to date14. It is highly attractive and challenging to develop useful strategies for the design and construction of efficient photocatalysts to increase the efficiency of the NOCM.
The loading of suitable co-catalysts and the manipulation of the nanostructure of a semiconductor photocatalyst are two key strategies to improve photocatalytic performance. Some noble metal co-catalysts such as Pt16, Ag18, Au19, and Pd20,21 have been exploited for the metal oxide-based photocatalytic NOCM. For example, a single-atom Pd-modified TiO2 catalyst (Pd1/TiO2) showed a high activity among different catalysts (Supplementary Table 1), and the accumulated photogenerated holes on Pd sites were proposed for the activation and conversion of CH4 to C2H621. Engineering the exposed facets of semiconductor nanocrystals is another useful strategy to tune the photocatalytic performance. The current consensus is that the facet with higher surface energy is more active in photocatalytic reactions23,24. As a representative example, the anatase TiO2 {001} facet with a higher surface energy has been proven to possess higher photocatalytic activity than the {101} facet with a lower surface energy25,26,27. Recently, such a facet effect has also been proven in photocatalytic CH4 conversions28,29. The {001}-dominated TiO2 showed a significantly higher CH3OH formation rate than the {101}-dominated TiO2 in photocatalytic CH4 oxidation by O228, confirming the role of high-energy surfaces. For the photocatalytic NOCM, a polar {001}-dominated ZnO was similarly beneficial to CH4 activation and C2H6 formation29, offering a C2H6 formation rate of about 10 µmol g−1 h−1.
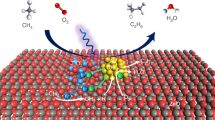
In the present work, we report our discovery of an unusual phenomenon that the anatase TiO2 nanocrystal mainly exposing the stable {101} facet shows the best performance, whereas that with the high-energy {001} facet is significantly less active and selective for the photocatalytic NOCM in the presence of water. The Pd co-catalyst also plays a crucial role in C2 formation, and it is unique that the positively charged Pd species, rather than the metallic Pd species accounts for the selective formation of C2 compounds. We achieve a CH4 conversion rate of 326 μmol g−1 h−1 with 81% selectivity of C2 compounds over {101}-dominated anatase TiO2 nanocrystals modified by Pd2+-containing co-catalyst, and the attained C2 formation rate represents one of the best values reported to date (Supplementary Table 1). Our studies unveil that this unusual finding is related to the location of the active species generated in photocatalysis. The •OH radicals formed in the liquid phase are proposed to work as the active species for the selective conversion of CH4 to C2H6, whereas those strongly adsorbed on the surface (in the case of {001}-dominant TiO2) cannot efficiently participate in CH4 activation or may lead to over-oxidation. Our studies further suggest that the Pd co-catalyst contributes to aiding the coupling of •CH3 radicals by enhancing their adsorption onto catalyst surfaces, besides enhancing the electron-hole separation.
Results
Characterizations of Pd-loaded TiO2 nanocrystals
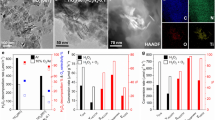
Three types of TiO2 nanocrystals with different morphologies and exposed facets were synthesized by hydrothermal synthesis30. Transmission electron microscopy (TEM) observations show that the obtained nanocrystals are in nanosheet, nanorod, and nanobipyramid morphologies (Supplementary Fig. 1a–c). High-resolution TEM (HRTEM) reveals that the TiO2 samples with nanosheet, nanorod, and nanobipyramid morphologies mainly expose facets of {001}, {100}, and {101}, respectively (Figs. 1a-1c). A detailed analysis of the TEM result indicates that the fraction of the mainly exposed facet exceeds 80% in each TiO2 sample (Supplementary Fig. 2). Hereafter, we denote these three nanocrystals as TiO2-{001}, TiO2-{100}, and TiO2-{101} for brevity. X-ray diffraction (XRD) and Raman spectroscopy both reveal that the three TiO2 nanocrystals are in the anatase phase (Supplementary Fig. 3a and 3b). The difference in the broadening of XRD peaks probably arises from the difference in the average sizes of TiO2 nanocrystals. Compared to TiO2-{101} and TiO2-{100}, the TiO2-{001} sample showed relatively stronger (200) and weaker (004) diffraction peaks (Supplementary Fig. 3a), in agreement with the TEM result that the TiO2-{001} sample has the largest side length in the [100] direction and the smallest thickness in the [001] direction31. The Eg bands at 144 and 636 cm−1 in Raman spectra are attributable to the symmetric stretching vibration of O−Ti−O of TiO2 (Ref. 31), and the weaker Eg bands for TiO2-{001} (Supplementary Fig. 3b) are consistent with the fact that this sample exposes a higher fraction of {001} facet with fewer symmetric stretching vibration modes of O−Ti−O. X-ray photoelectron spectroscopy (XPS) measurements confirm that the TiO2 nanocrystals are composed of only Ti and O elements without other impurities and that the oxidation states of Ti or O are the same in the three samples (Supplementary Fig. 3c–3e).
Pd species were loaded onto TiO2 by an adsorption-reduction method and the measured contents of Pd loaded on TiO2-{001}, TiO2-{100}, and TiO2-{101} were almost the same (Supplementary Table 2). The morphologies and the crystalline structures of the three TiO2 samples did not change after loading Pd (Supplementary Fig. 1d–f and Supplementary Fig. 3f). The scanning transmission electron microscopy (STEM) showed that the average sizes of Pd loaded on the TiO2-{101}, TiO2-{100}, and TiO2-{001} were 2.2–2.5 nm (Fig. 1d and Supplementary Fig. 4a, 4b). The HRTEM observation implies the non-crystalline cluster feature of the loaded Pd (Fig. 1e). Broad Pd 3d XPS spectra, which could be deconvoluted into two components of Pd0 and Pd2+ (Ref. 32), were observed over the three TiO2 surfaces (Fig. 1f and Supplementary Fig. 4c, 4d). The fractions of Pd2+ estimated from the XPS spectra were also similar and close to 50% over the three Pd/TiO2 samples (Supplementary Table 2). Furthermore, the fractions of Pd2+ on the Pd/TiO2 catalysts basically kept unchanged after 4 h of photocatalytic NOCM reaction (Supplementary Fig. 5 and Supplementary Table 3). The high fraction of Pd2+ on our Pd/TiO2 catalysts and the keeping of Pd2+ during the photocatalytic reaction could be attributed to the strong interaction between the small Pd nanoparticles or nanoclusters and TiO233,34.
Photocatalytic properties of Pd-loaded TiO2 nanocrystals
The photocatalytic CH4 conversion was performed in a batch reactor in the presence of H2O at ambient temperature and pressure, and C2H6, C2H4, CO2, and H2 were detected over our Pd/TiO2 catalysts. The performance was found to depend strongly on the exposed facets of TiO2; the Pd/TiO2-{101} catalyst demonstrated the best performance (Fig. 2a). Over this catalyst, the amounts of C2H6, C2H4, CO2, and H2 formed after 4 h of irradiation were 10, 0.16, 4.8, and 42 μmol, respectively (Supplementary Table 4). The ratio of the amount of holes consumed in the formation of C2H6, C2H4, and CO2 via CH4 oxidation to that of electrons consumed in the formation of H2 was calculated to be close to 1.0, confirming that the photogenerated hole and electrons participate in the formation of carbon-containing products and H2, respectively. Control experiments showed no formation of carbon-containing products and a significantly decreased amount of H2 by using N2 instead of CH4 (Supplementary Table 5). We only detected a trace amount of O2 in the absence of CH4, suggesting that the oxidation of H2O to O2 by photogenerated holes proceeded much slower, and this also decelerated the half-reaction of H2 evolution by photogenerated electrons. No products were detected without light irradiation or in the absence of a catalyst. All these facts verify that the carbon-containing products originate from CH4 via light-driven photocatalytic reactions.
a Effect of exposed anatase facet on NOCM performances over Pd/TiO2 catalysts. b Time courses for CH4 conversion and C2 selectivity over Pd/TiO2 catalysts with different exposed anatase facets. c Effect of Pd content on NOCM performances over Pd/TiO2-{101} catalysts. d Effect of the percentages of Pd2+ on Pd nanoparticles on NOCM performances and transient photocurrent densities over Pd/TiO2-{101} catalysts. Reaction conditions: catalyst, 0.020 g; water, 50 mL; CH4, 45 mL (2009 μmol); light source, 300 W Xe lamp (λ = 320-780 nm). The error bar represents the relative deviation, which is within 5%.
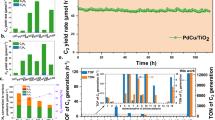
A more detailed analysis shows that the Pd/TiO2-{101} catalyst, which shows the best performance, offers a C2H6 formation rate of 262 μmol g−1 h−1, which is 3.7 and 11 times larger than those for the Pd/TiO2-{100} and Pd/TiO2-{001} catalysts (Fig. 2a). The C2 selectivity is also the highest over the Pd/TiO2-{101} catalyst (81%) and decreases in the sequence of Pd/TiO2-{101} > Pd/TiO2-{100} > Pd/TiO2-{001}. The CH4 conversion increased almost linearly with the reaction time over each catalyst, suggesting that the reaction proceeded steadily, and the values were 1.9%, 0.94%, and 0.75% over the Pd/TiO2-{101}, Pd/TiO2-{100}, and Pd/TiO2-{001} catalysts, respectively, after 8 h of reaction (Fig. 2b). Meanwhile, the C2 selectivity only changed slightly with the reaction time and the values over the Pd/TiO2-{101}, Pd/TiO2-{100}, and Pd/TiO2-{001} catalysts remained at about 80%, 70%, and 30%, respectively, indicating that the C2 products and CO2 were formed in parallel from CH4. We evaluated the specific C2 formation rate on the basis of the surface area of TiO2, and the values were 5.3, 0.74, and 0.37 µmol m−2 h−1 for the Pd/TiO2-{101}, Pd/TiO2-{100}, and Pd/TiO2-{001}, respectively (Supplementary Table 6). Further, we have compared the photocatalytic NOCM performance of the Pd/TiO2-{101} catalyst with that of a Pd-promoted commercial P25 catalyst, which is known to possess high efficiencies in photocatalysis. The C2 selectivity and yield of our Pd/TiO2-{101} catalyst are significantly higher than those of Pd/P25 (Supplementary Table 4). These results demonstrate the intrinsic superiority of the {101} facet of TiO2 in photocatalytic NOCM. Therefore, the TiO2-catalyzed NOCM is a strong structure-sensitive reaction, and the exposed facet of TiO2 is not only the CH4 conversion but also the selectivity of C2 compounds. We have measured the apparent quantum yield (AQY) of the Pd/TiO2-{101} catalyst for the formation of C2H6 and C2H4, and the AQY value at a wavelength of 365 nm is 0.21%.
Our studies reveal that the Pd co-catalyst also plays a crucial role in accelerating the formation of C2 compounds. In the absence of Pd co-catalyst, the formation rates of C2 compounds and H2 were both very low. The presence of Pd even with a very low content (0.013 wt%) could remarkably accelerate the formation of either C2 compounds or H2, and the C2 selectivity was also significantly enhanced at the same time (Fig. 2c). A further increase in the Pd content up to 0.78 wt% increased the C2+ selectivity and formation rate, although the H2 formation was not significantly accelerated (Fig. 2c). It is noteworthy that the Pd/TiO2-{101} catalyst displays a higher C2 formation rate and C2 selectivity than the Pd/TiO2-{100} and Pd/TiO2-{001} catalysts irrespective of the Pd content (Fig. 2c and Supplementary Fig. 6). We prepared Cl−-free Pd/TiO2-{101} catalyst by using Pd(NO3)2 as the Pd precursor instead of PdCl2, which was typically used in the present work, and the XPS measurement confirmed the absence of chlorine on the surface of this catalyst (Supplementary Fig. 7). The Pd/TiO2-{101} and the Cl−-free Pd/TiO2-{101} catalysts exhibited similar performances for photocatalytic NOCM (Supplementary Table 4), excluding the effect of Cl− residues. The decoration of TiO2-{101} with other noble metal or transition metal co-catalysts including Pt, Au, Ag, Cu, or Fe could also promote the formation of C2 compounds, but these co-catalysts except for Pt, showed remarkably poorer performances than Pd (Supplementary Fig. 8a). Pt was another highly efficient co-catalyst for C2 formation, and the enhancing effect of Pt was only slightly inferior to that of Pd. The comparison of Pt-decorated TiO2 catalysts with different exposed facets shows that the formation rate and selectivity of C2 compounds decrease in the same sequence with the Pd-decorated series of catalysts, i.e., Pt/TiO2-{101} > Pt/TiO2-{100} > Pt/TiO2-{001} (Supplementary Fig. 8b). This demonstrates once again that the TiO2 {101} facet, which is the most stable and has been regarded as the least active facet in photocatalysis25,26,27, shows the promising performance for the photocatalytic NOCM in the presence of a suitable co-catalyst.
We found that the oxidation state of the Pd co-catalyst exerted a significant effect on the photocatalytic NOCM. The method for post-treating the catalyst precursor, i.e., PdCl2 adsorbed on TiO2, could regulate the Pd oxidation state but keep the Pd content the same in the final catalyst. The H2 reduction of the calcined PdCl2/TiO2-{101} precursor offered the Pd/TiO2-{101} catalyst, which is typically adopted in the present work and has a surface Pd2+/Pd0 ratio close to 1.0 (Supplementary Table 2). On the other hand, the heat treatment in air and the reduction with NaBH4 of the PdCl2/TiO2-{101} precursor offered catalysts (denoted as Pd-air/TiO2-{101} and Pd-NaBH4/TiO2-{101}) with larger and smaller surface Pd2+/Pd0 ratios, respectively (Supplementary Fig. 9). The fractions of Pd2+ on the surfaces of Pd-air/TiO2-{101}, Pd/TiO2-{101}, and Pd-NaBH4/TiO2-{101} were evaluated to be 61%, 49%, and 33%, respectively by XPS (Supplementary Fig. 9). The catalytic study reveals that a higher surface fraction of Pd2+ results in a higher C2 formation rate and higher C2 selectivity (Fig. 2d). It is well known that the noble metal co-catalyst can accelerate the charge separation by accepting the photogenerated electrons from the semiconductor35. Our transient photocurrent response measurements showed that the photocurrent density decreased with an increase in the fraction of Pd2+ (Fig. 2d and Supplementary Fig. 10a), indicating that Pd0 plays a crucial role in accelerating the electron-hole separation. The control experiment for H2 evolution in the presence of CH3OH as a sacrificial agent showed that the H2 evolution decreased upon increasing the fraction of Pd2+ (Supplementary Fig. 10b). Therefore, we propose that Pd0 accelerated the electron-hole separation and is responsible for H2 formation, whereas Pd2+ accounts for the formation of C2 compounds in our system.
Structure-property relationship and reaction mechanism
Our characterizations show that there are no significant differences in the light absorption and the energy-band structure among the three Pd/TiO2 catalysts with different exposed facets (Fig. 3a and Supplementary Fig. 11). The electron-hole separation abilities of the Pd/TiO2-{101} and Pd/TiO2-{001} catalysts are also similar, although such ability of the Pd/TiO2-{100} catalyst is lower (inset of Fig. 3a and Supplementary Fig. 12a). The photocatalytic H2 evolution decreased in the sequence of Pd/TiO2-{001} > Pd/TiO2-{101} > Pd/TiO2-{100} (Supplementary Fig. 12b). This trend of H2 evolution is different from that for photocatalytic NOCM and could be explained by the higher electron-hole separation abilities of Pd/TiO2-{001} and Pd/TiO2-{101} (inset of Fig. 3a) and the higher surface energy of TiO2-(001)25. The density of oxygen vacancies, which might determine the surface reactivity, was measured quantitatively by electron titration27, and it followed a trend of Pd/TiO2-{001} > Pd/TiO2-{101} > Pd/TiO2-{100} (Supplementary Fig. 13). This trend did not correlate well with that for the specific C2H6 formation rate and C2 selectivity. Further, the surface area of the Pd/TiO2-{101} catalyst with the best C2 formation performance is the lowest (Supplementary Table 6). Therefore, it is not the shape-based parameters that may affect the light absorption, the electron-hole separation, and the surface area or the density of oxygen vacancies, but the exposed facet-based factor (not just the surface energy) plays a determining role in the TiO2-catalysed NOCM.
a UV-Vis diffuse reflectance spectra and transient photocurrent responses (inset) for Pd/TiO2 catalysts with different exposed anatase facets. b In situ ESR spectra using DMPO as a trapping agent under light irradiation for Pd/TiO2 catalysts with different exposed anatase facets. c The relationship between C2H6 formation rate and concentration of •OH measured by the fluorescence method using terephthalic acid as a probe molecule. d The relationship between C2H6 selectivity and percentage of liquid-phase •OH. The inset of Fig. 3d displays the •OH concentrations in the absence and presence of fluoride ions. The error bar represents the relative deviation, which is within 5%.
Our catalytic result already implied that photogenerated holes and electrons functioned for the oxidative conversion of CH4 and the reductive formation of H2, respectively. We further found the crucial role of the presence of H2O in the formation of C2 compounds; the C2 formation rate over the Pd/TiO2-{101} catalyst was very low without H2O and the presence of H2O increased the C2 formation rate for ~29 times (Supplementary Fig. 14). The substitution of H2O by dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), or perfluorohexanes as the solvent resulted in no formation of C2 products, further pointing out the determining role of H2O. When terephthalic acid (25 μmol), a scavenger of •OH radicals36,37, was added to the photocatalytic system with the Pd/TiO2-{101} catalyst, the amounts of C2H6, C2H4, and CO2 formed after 2 h of irradiation decreased drastically from 6.4, 0.16, and 3.1 μmol to 0.12, 0, and 0.28 μmol, respectively. We propose that the •OH radical formed in the presence of H2O may participate in the activation of CH4 and the formation of C2 compounds.
The electron spin resonance (ESR) spectroscopic studies using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a radical trapping agent showed a quartet spectrum corresponding to a DMPO-OH spin adduct38, under photocatalytic conditions, confirming the generation of •OH radicals (Fig. 3b). The fluorescence measurements using terephthalic acid as a probe molecule could quantify •OH radicals in the liquid phase (Supplementary Fig. 15a-c and Fig. 3c)36,37. Both the ESR and the fluorescence measurements reveal that the concentration of •OH radicals in the liquid phase decreases in the sequence of Pd/TiO2-{101} > Pd/TiO2-{100} > Pd/TiO2-{001}. This trend correlates well with that for C2H6 formation rate (Fig. 3c). We further performed surface fluorination to measure the total amount of •OH radicals generated (Supplementary Fig. 15d-f and inset of Fig. 3d), considering that •OH radicals may either be adsorbed on catalyst surfaces or exist in liquid phase and the addition of fluoride ions could release the •OH adsorbed on the surface into the solution39,40. The ratio of the •OH radicals existing in the liquid phase in all the •OH radicals generated could be evaluated and was found to depend on the exposed facet of TiO2 (Fig. 3d). Such a ratio was the highest for the Pd/TiO2-{101} catalyst. It is of interest that the ratio of the •OH in the liquid phase correlates well with the C2 selectivity for the catalysts with different exposed facets of TiO2 (Fig. 3d). These results enable us to propose that the •OH radicals existing in the liquid phase rather than adsorbed on catalyst surfaces are responsible for the formation of C2 compounds.
Density functional theory (DFT) calculations were performed to gain in-depth insights into the reaction mechanism. We compared the adsorption strength of •OH radicals on three model surfaces of anatase, i.e., {001}, {100}, and {101} surfaces (Supplementary Fig. 16). Our calculations show that the adsorption energy of •OH radicals follows the trend of {001} (−1.57 eV) < {101} (−0.97 eV) < {100} (−0.72 eV) (Fig. 4a and Supplementary Fig. 17). Such strong adsorption indicates that the direct desorption of •OH from the anatase surface is energetically unfavorable. Alternatively, the •OH in the liquid phase may be generated by the reaction between the adsorbed •OH and a H2O molecule near the surface via H transfer (inset of Fig. 4a). Our calculations show that such a H transfer reaction is exothermic and the reaction energies for the {001}, {100}, and {101} surfaces are −0.04, −0.16, and −0.22 eV, respectively (Fig. 4a and Supplementary Fig. 17). This result indicates that the liquid-phase •OH radicals would be formed more facilely in the case of TiO2-{101} surface as compared to the other two surfaces and agrees well with the experimental fact that the fraction of liquid-phase •OH radicals is the highest for the Pd/TiO2-{101} catalyst (Fig. 3d).
a The liquid-phase •OH formation energy versus the •OH adsorption energy on anatase TiO2 with different exposed facets. The inset of Fig. 4a displays the optimized structures for •OHliquid-phase generation by H-transfer on TiO2-{101} surface. b The relative energy for the coupling of •CH3 to C2H6 on the model surfaces of Pd1, Pd4, and Pd4O loaded on anatase TiO2-{101} as well as on the Pd(111) surface. The inset of Fig. 4b displays the optimized structures for •CH3 enrichment and self-coupling on Pd4O site loaded on TiO2-{101}. c Mechanism for photocatalytic NOCM with H2O on Pd/TiO2-{101}.
Our DFT calculations further reveal that the •OH radicals either adsorbed on the TiO2-{101} surface or existing in liquid phase are capable of activating CH4 via H abstraction to generate •CH3 by overcoming barriers of 0.14 and 0.02 eV, respectively (Supplementary Fig. 18). On the other hand, the barrier for the cleavage of the C − H bond in CH4 on the Pd4O cluster over a model Pd4O/TiO2-{101} catalyst is 0.53 eV (Supplementary Fig. 18). Thus, the •OH radical would be more favorable for initiating the C − H bond cleavage in our system. The selectivity of the whole reaction is determined by the fate of •CH3. Considering the low concentration of •CH3 radicals in the liquid phase, the probability of self-coupling of two liquid-phase •CH3 radicals would be very limited. The encounter of •CH3 radicals with other •OH radicals in liquid phase will cause the formation of a C − O bond rather than C − C bond, eventually leading to CO2. Our DFT calculations reveal that the reaction between •CH3 and H2O, which leads to the formation of CH3OH, is a thermodynamically unfavorable reaction (∆G = +1.13 eV). A couple of studies have proposed that the metal co-catalyst may adsorb •CH3 radicals and catalyze their subsequent transformations12,20,41. To further understand the potential role of Pd co-catalysts in the coupling of •CH3 radicals, we performed DFT calculations using four models of Pd co-catalysts. The result shows that the isolated Pd1 has the ability to trap only one •CH3 radical (Fig. 4b), unfavorable for the coupling that needs two adjacent •CH3 radicals. On the other hand, the DFT calculations demonstrate that adjacent •CH3 radicals could be enriched on Pd(111) surface, supported Pd4 cluster, and supported Pd4O cluster with Pd−Pd pair sites from the viewpoint of thermodynamics (Fig. 4b). The energy barriers for the formation of C2H6 via coupling of the •CH3 radicals adsorbed on Pd(111) surface, Pd4 cluster, and Pd4O cluster are calculated to be 1.93, 0.99, and 0.48 eV, respectively (Fig. 4b and Supplementary Fig. 19). Therefore, both the cluster feature of Pd co-catalysts and the presence of positively charged Pdδ+ sites play crucial roles in C2 formation by affecting the enrichment and the coupling of •CH3 radicals. Experimentally, when a physical mixture of the TiO2-{101} sample and a Pd/Al2O3 catalyst, which itself was inactive for photocatalytic NOCM, was used instead of the Pd/TiO2-{101} catalyst, CH4 could be converted into C2H6 with 53% selectivity (Supplementary Table 4). This supports the result obtained from DFT calculations that the Pd co-catalyst with Pd2+ contributes to aiding the coupling by trapping •CH3 radicals from the liquid phase.
Based on the above experimental and the DFT calculation results, we propose that the photocatalytic NOCM with H2O proceeds via a heterogeneous-homogeneous mechanism over the Pd/TiO2-{101} catalyst (Fig. 4c). The generation of •OH radicals by the interaction of H2O with photogenerated holes has been observed. Our studies reveal that a part of •OH radicals are present in the liquid phase, and these •OH radicals are probably formed by the H transfer between the •OH radicals formed on semiconductor surfaces and the liquid-phase H2O near the surface. The structure-property correlation suggests that the •OH radicals in the liquid phase participate in the activation of CH4 via H abstraction to form •CH3. The Pd nanoparticles may function for catching •CH3 radicals from the liquid phase and the presence of Pd2+ sites on the nanoparticles favors the coupling of •CH3 radicals by lowering the energy barrier. It is noteworthy that the Pd/TiO2-{101} catalyst catalyzes the generation of liquid-phase •OH radicals more facilely as compared to other catalysts with different exposed facets, leading to the remarkable facet effect for the formation of C2 compounds.
Discussion
This work presents an efficient photocatalytic system for NOCM in the presence of H2O, achieving a CH4 conversion rate of 326 μmol g−1 h−1 at 81% C2 selectivity, which represents one of the best values reported to date. We discovered a unique facet effect that the anatase TiO2 nanocrystal mainly exposing {101} facet, which is usually considered as less active in photocatalysis, shows a significantly higher formation rate of C2 compounds, whereas that mainly exposing high-energy {001} facet is rather less active and selective. The formation energy of •OH radicals in the liquid phase and the surface fraction of Pd2+ are two key descriptors in the present system. It is revealed that the exposed facet can control the concentration of •OH radicals existing in the liquid phase during photocatalysis, and the anatase {101} facet offers a significantly higher concentration of liquid-phase •OH radicals than the corresponding {001} and {100} facets. The liquid-phase •OH radical is proposed to be formed by the H transfer between the •OH radical adsorbed on the TiO2 surface and a H2O molecule near the surface. A higher ratio of •OH radicals in the liquid phase to those on TiO2 surfaces leads to higher C2 selectivity, suggesting that the •OH radicals in the liquid phase account for the selective conversion of CH4 to C2 compounds, whereas those adsorbed on TiO2 surfaces may contribute to the CO2 formation. The Pd co-catalyst not only accelerates the electron-hole separation but also plays a role in enhancing the coupling of •CH3 radicals by adsorbing and enriching the •CH3 radicals from the liquid phase. The presence of positively charged Pd2+ sites on the Pd nanoparticles is beneficial to lowering the energy barrier for the coupling of •CH3 radicals accumulated on Pd nanoparticles.
Methods
Synthesis of catalysts
TiO2-{101} nanocrystals were synthesized by a hydrothermal method. In brief, titanium isopropoxide (20 mL) and ultra-pure water (2.0 mL) were added into 100 mL Teflon-lined autoclave and mixed thoroughly by stirring, followed by hydrothermal treatment at 200 °C for 24 h. After the hydrothermal treatment, a white solid product was collected by centrifugation and repeated washing with water and ethanol. The TiO2-{101} was obtained by calcining the solid product in air at 500 °C for 3 h.
TiO2-{100} nanocrystals were synthesized by the following two-step method. In the first step, TiCl4 (6.6 mL) was added into 20 mL HCl aqueous solution (0.43 M) at 0 °C. After stirring for 30 min, an NH3 aqueous solution (5.5 wt%) was added, and then the pH value was adjusted to around 6.5 using an NH3 aqueous solution (4.0 wt%). A white suspension was obtained and stirred for 2 h, followed by repeatedly washing until chloride ions were completely removed. A solid Ti(OH)4 precursor was collected after drying at 70 °C for 12 h. In the second step, Ti(OH)4 (2.0 g) was dispersed into a mixed solution of (NH4)2SO4 (0.50 g), water (15 mL), and isopropanol (15 mL). After being stirred for 1 h, the mixture was transferred into an autoclave (100 mL) and was subjected to hydrothermal treatment at 180 °C for 24 h. After hydrothermal synthesis, the solid product was collected by centrifugation, followed by repeated washing with water and ethanol and drying at 60 °C.
TiO2-{001} nanocrystals were synthesized by a procedure reported previously30. Hydrofluoric acid (40 wt%) was employed as a capping agent. Tetrabutyl titanate (25 mL) and HF (3.0 mL) were mixed thoroughly to form a homogeneous solution and were subjected to hydrothermal treatment in an autoclave at 200 °C for 24 h. After hydrothermal synthesis, the solid product was collected by centrifugation, followed by repeated washing with water and ethanol. The sample was further dispersed in an aqueous solution of NaOH (0.1 M), followed by stirring for 24 h. The solid powders were recovered by centrifugation and washed with water to neutral, followed by drying at 60 °C.
The Pd/TiO2 catalysts were prepared by an adsorption-reduction method. Typically, a PdCl2 aqueous solution (concentration, 1.25 mg mL−1; volume, 1.14 mL) was added dropwise into a mixture of TiO2 nanocrystals synthesized above (100 mg) and water (30 mL). The pale-yellow solid with PdCl2 adsorbed on TiO2 (PdCl2/TiO2) was obtained after removal of H2O. The obtained powdery precursor was calcined in air at 300 °C for 2 h and was reduced in H2 flow at 300 °C for 2 h. The TiO2 catalysts loaded with other metal co-catalysts, including Pt, Au, Ag, Cu, and Fe, were prepared by the same procedures except for using H2PtCl6, HAuCl4, AgNO3, Cu(NO3)2, and Fe(NO3)3 as the metal precursors instead of PdCl2.
To investigate the effect of the fraction of Pd2+ or the ratio of Pd2+/Pd0, we also prepared Pd-air/TiO2-{101} and Pd-NaBH4/TiO2-{101} besides the standard Pd/TiO2-{101} catalyst prepared by the H2 reduction of the calcined PdCl2/TiO2. The Pd-air/TiO2-{101} was obtained by calcining the PdCl2/TiO2 precursor at 300 °C for 2 h. The Pd-NaBH4/TiO2-{101} catalyst was obtained by reducing the PdCl2/TiO2 precursor with an aqueous solution of sodium borohydride (0.1 M).
Photocatalytic reaction
Prior to each photocatalytic reaction, the catalyst (20 mg) was ultrasonically dispersed in ultra-pure water (50 mL) in a batch-type reactor (95 mL). The system was first evacuated and then purged with CH4 (99.999%) for 10 min at a constant pressure. The reaction was performed by irradiation under a 300 W Xe lamp (λ = 320-780 nm) for 4 h at room temperature. After the reaction, potential gaseous products (including C2H6, C2H4, C3H8, C3H6, CO2, O2, and H2) were detected using a high-speed micro gas chromatograph (INFICON Micro GC Fusion) equipped with molecular sieve 5 A and Q-bond columns as well as a high-sensitivity thermal conductivity detector. Potential liquid organic products (including CH3OH, HCHO, HCOOH) were quantitatively analyzed by 1H nuclear magnetic resonance (NMR) spectroscopy (Advance III 500-MHz Unity plus spectrometer, Bruker). Our analysis showed that the major products were C2H6, C2H4, H2, and CO2. No C3 gaseous products or liquid organic compounds were observed in our photocatalytic system. The formation rates of the carbon-containing products were calculated on a carbon basis.
The performance parameters were calculated according to the following equations:
where n, m, and t represent the amount of substance, mass of the catalyst, and reaction time.
The ratio of electrons and holes consumed in the reaction process was calculated using the following equation.
We measured the apparent quantum yield (AQY) of the Pd/TiO2-{101} catalyst by using LED light for the photocatalytic NOCM to C2H6 and C2H4. The AQY for the formation of C2H6 and C2H4 was calculated using the following equation:
where n, NA, I, S, and t represent the molar amounts of C2H6 and C2H4, Avogadro’s constant, light intensity (0.20 W cm−2), irradiation area (0.78 cm2), and reaction time (3600 s), respectively. Eλ is calculated using hc/λ (λ = 365 nm).
Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultima IV diffractometer. Raman spectroscopy measurements were carried out on a Renishaw inVia Raman microscope. Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) measurements were performed on a Tecnai F20 electron microscope (Phillips Analytical). X-ray photoelectron spectroscopy (XPS) was conducted using a Qtac-100 instrument, and all samples were under the protection of N2 before XPS measurements. The content of each element was measured with an inductively coupled plasma optical emission spectrometer (ICP-OES, SPECTROBLUE FMX36). UV-Vis diffuse reflectance spectroscopy was measured on a Varian Cary 5000 spectrophotometer. Electron spin resonance (ESR) spectroscopic measurements were performed on a Bruker EMX-10/12 ESR spectrometer at room temperature. Photoluminescence spectra were recorded on a fluorescence spectrophotometer (F4500). N2 physisorption measurements were performed on a Micromeritics Tristar 3020 surface area analyzer.
Measuring liquid-phase •OH radicals: The Pd/TiO2 catalyst (20 mg) was dispersed in a 50 mL aqueous solution containing terephthalic acid (5.0 × 10−4 M) and NaOH (2.0×10−3 M). After stirring for 30 min in the dark, the suspension was irradiated under a 300 W Xe lamp (λ = 320-780 nm). A small portion of the suspension (1.0 mL) was taken out and filtered after 0, 10, 20, and 30 min of irradiation. The filtrate (0.5 mL) was mixed with water (2.5 mL), and the solution was measured on a fluorescence spectrophotometer (F4500) at an excitation wavelength of 315 nm. Both the excitation and emission slit widths were 2.5 nm, and the scan speed was 1200 nm min−1.
Measuring total •OH radicals: The method adopted for measuring the concentration of total •OH radicals was the same as that for measuring the liquid-phase •OH, except for using Pd/TiO2-F instead of the original Pd/TiO2. The Pd/TiO2-F was prepared by the following procedure. An HF aqueous solution (40 wt%, 50 μL) was added to a mixture composed of Pd/TiO2 (50 mg) and water (20 mL) under stirring, and the suspension was further stirred at room temperature for 4 h. It is well known that the hydroxyl groups on the surface of TiO2 can be completely replaced by fluoride ions at a pH of 3-4. Surface fluorination can promote the desorption of surface-bound •OH radicals into the liquid phase. Therefore, the •OH radicals generated using Pd/TiO2-F represent the concentration of total •OH radicals.
The density of oxygen vacancies was measured quantitatively by electronic titration using thionine acetate as a titrant27. Typically, the Pd/TiO2 catalyst (5.0 mg) was ultrasonically dispersed in ultra-pure water (50 mL) in a batch-type reactor (95 mL). The system was first evacuated and then purged with CH4 (99.999%) for 10 min at a constant pressure. The reaction was performed by irradiation under a 300 W Xe lamp (λ = 320-780 nm) for 2 h at room temperature. After the light irradiation, an aqueous solution of thionine acetate (0.3 mM, 1 mL) was quickly added to the suspension. The resulting suspension was filtrated, and the UV-vis absorption of the filtrate at 602.5 nm was measured. By comparing with the standard curve of different concentrations of thionine acetate aqueous solutions, the corresponding amount of thionine acetate consumed can be obtained. The density of oxygen vacancies was evaluated according to the correspondence that one oxygen vacancy is accompanied by two Ti3+, and the oxidation of 2 moles of Ti3+ requires 1 mole of thionine acetate27.
The transient photocurrent response measurements were performed on an electrochemical workstation (CHI760E) in a standard three-electrode system. The Pt electrode and Ag/AgCl electrode were used as the counter and reference electrode, respectively. The Na2SO4 solution (0.5 mol/L) was used as the electrolyte. The working electrode was prepared as follows: the catalyst (10 mg) was dispersed in a mixture solution of Nafion perfluorinated resin solution (10 μL) and ethanol (1 mL). 100 μL of dispersion was uniformly dropped on the FTO plate (1 cm × 2 cm) so that the coating area was 1 cm−2. An LED lamp with a wavelength of 365 nm served as a light source. The applied potential was 0 V vs. the Ag/AgCl reference electrode.
Computational methods
The first principle spin-polarized calculations were carried out by using Vienna ab initio simulation package 6.3 (VASP 6.3)42,43. The exchange-correlation functional was described by generalized-gradient approximation (GGA) in the version of Perdew–Burke–Ernzerhof (PBE)44. The projector augmented-wave method (PAW)45 was used to describe the ion-electron interaction with a plane wave basis set using a cut-off energy of 450 eV. As displayed in Supplementary Fig. 16, the anatase TiO2-{001}, TiO2-{100}, and TiO2-{101} surfaces were modeled using a six-layer slab with (3 × 3) surface supercell (Ti54O108), a seven-layer slab with (3 × 1) surface supercell (Ti42O84), and an eight-layer slab with (2 × 3) surface supercell (Ti48O96), respectively. A 15 Å thick vacuum layer along the Z direction was adopted to avoid the interaction between adjacent images. To simulate the loaded Pd species with different valence states, the most stable Pd4 cluster and Pd4O cluster were deposited on the TiO2-{101} surface. For comparison, a single-atom Pd catalyst, i.e., Pd1/TiO2-{101}, was also constructed by replacing one Ti atom with Pd atom and removing one adjacent two-coordinated oxygen atom. The Pd-{111} surface was modeled with a 4 × 4 supercell and 4 layers.
During the structural optimization, the upper two layers with adsorbates were allowed to be fully relaxed while the other layers were fixed. The Brillouin zone of slab model was sampled by a Monkhorst−Pack scheme46 with a grid of 3 × 3 × 1 k points. The Grimme’s dispersion correction (DFT-D3) was utilized to include the van der Waals interaction47. The conjugate gradient method was used to optimize the positions of the ions until the residual force on each ion was less than 0.02 eV Å−1. The total energy had been calculated up to an accuracy of 10−4 eV.
Generally speaking, PBE has the tendency to delocalize unpaired electrons due to the inherent self-interaction. To alleviate this issue, we used the DFT + U method48 where a Hubbard-type correction was applied on both Ti-d and O-p orbitals. It was previously reported that U(d) = 4.2 eV for Ti and U(p) = 6.3 eV for O could be used to describe the polaronic states of TiO249. The spin density difference was utilized to identify a hole that was localized at the OH group. To locate the transition states (TSs), the climbing-image nudged elastic band method (CI-NEB) was applied with the convergence criteria of force of 0.05 eV Å−1 for each atom50.
The adsorption energy (∆Gads) was defined using the following equation:
where G(adsorbate/surface), G(adsorbate), and G(surface) the free energies of the combined system, the gas-phase adsorbates, and the clean surface, respectively.
The liquid-phase •OH formation energy was calculated using the following equation:
To calculate the reaction between •CH3 and H2O, leading to the formation of CH3OH, we adopted the advanced Gaussian-3(G3) computational method with Gaussian 16 code51,52, which is known for its accuracy in estimating molecular energies. We conducted studies using the B3LYP functional combined with the 6-311 G(d) basis set to perform geometry optimization and frequency calculation. Single-point energy calculation was performed at G3(MP2) level. The relevant chemical equation is:
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this work are available in the manuscript. Source data are provided in this paper.
References
Crabtree, R. H. Aspects of methane chemistry. Chem. Rev. 95, 987–1007 (1995).
Tang, P., Zhu, Q., Wu, Z. & Ma, D. Methane activation: the past and future. Energy Environ. Sci. 7, 2580–2591 (2014).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Zhang, T. Recent advances in heterogeneous catalysis for the nonoxidative conversion of methane. Chem. Sci. 12, 12529–21545 (2021).
Yuliatiw, L. & Yoshida, H. Photocatalytic conversion of methane. Chem. Soc. Rev. 37, 1592–1602 (2008).
Wu, X. et al. Solar-energy-mediated methane conversion. Joule 3, 1606–1636 (2019).
Meng, X. et al. Direct methane conversion under mild condition by thermo-, electro-, or photocatalysis. Chem 5, 2296–2325 (2019).
Xie, S. et al. Photocatalytic and electrocatalytic transformations of C1 molecules involving C–C coupling. Energy Environ. Sci. 14, 37–89 (2021).
Lin, X. et al. Methane conversion over artificial photocatalysts. Catal. Commun. 159, 1006346 (2021).
Li, Q. et al. Photocatalytic conversion of methane: recent advancements and prospects. Angew. Chem. Int. Ed. 61, e202108069 (2022).
Li, X., Wang, C. & Tang, J. Methane transformation by photocatalysis. Nat. Rev. Mater. 7, 617–632 (2022).
Jiang, Y., Fan, Y., Li, S. & Tang, Z. Photocatalytic methane conversion: insight into the mechanism of C(sp3)–H bond activation. CCS Chem. 5, 30–54 (2023).
Wu, X., Zhang, H., Xie, S. & Wang, Y. Photocatalytic conversion of methane: catalytically active sites and species. Chem. Catal. 3, 100437 (2023).
Wu, S., Wang, L. & Zhang, J. Photocatalytic non-oxidative coupling of methane: recent progress and future. J. Photochem. Photobiol. C.-Photochem. Rev. 46, 100400 (2021).
Li, L. et al. Efficient sunlight-driven dehydrogenative coupling of methane to ethane over a Zn+-modified zeolite. Angew. Chem. Int. Ed. 50, 8299–8303 (2011).
Wu, S. et al. Ga-doped and Pt-loaded porous TiO2-SiO2 for photocatalytic nonoxidative coupling of methane. J. Am. Chem. Soc. 141, 6592–6600 (2019).
Chen, Z. et al. Non-oxidative coupling of methane: N-type doping of niobium single atoms in TiO2-SiO2 induces electron localization. Angew. Chem. Int. Ed. 60, 11901–11909 (2021).
Yu, X. et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 5, 511–519 (2020).
Jiang, J., Ma, Y., Wu, X., Jiang, Y. & Hu, Y. H. Highly efficient light-driven methane coupling under ambient conditions based on an integrated design of a photocatalytic system. Green. Chem. 22, 4669–4675 (2020).
Jiang, W. et al. Pd-modified ZnO-Au enabling alkoxy intermediates formation and dehydrogenation for photocatalytic conversion of methane to ethylene. J. Am. Chem. Soc. 143, 269–278 (2021).
Zhang, W. et al. High-performance photocatalytic nonoxidative conversion of methane to ethane and hydrogen by heteroatoms-engineered TiO2. Nat. Commun. 13, 2806 (2022).
Wu, X. et al. Photocatalytic transformation of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 49, 6198–6223 (2020).
Liu, G., Yu, J. C., Lu, G. Q. & Cheng, H. M. Crystal facet engineering of semiconductor photocatalysts: motivations, advances and unique properties. Chem. Commun. 47, 6763–6783 (2011).
Jing, L., Zhou, W., Tian, G. & Fu, H. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 42, 9509–9549 (2013).
Yang, H. G. et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638–641 (2008).
Ong, W. et al. Highly reactive {001} facets of TiO2-based composites: synthesis, formation mechanism, and characterization. Nanoscale 6, 1946–2008 (2014).
Wu, X. et al. Selectivity control in photocatalytic valorization of biomass-derived platform compounds by surface engineering of titanium oxide. Chem 6, 3038–3053 (2020).
Feng, N. et al. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 12, 4652 (2021).
Meng, L. et al. Gold plasmon-induced photocatalytic dehydrogenative coupling of methane to ethane on polar oxide surfaces. Energy Environ. Sci. 11, 294–298 (2018).
Fu, C. et al. Site sensitivity of interfacial charge transfer and photocatalytic efficiency in photocatalysis: methanol oxidation on anatase TiO2 nanocrystals. Angew. Chem. Int. Ed. 60, 6160–6169 (2021).
Tian, F., Zhang, Y., Zhang, J. & Pan, C. Raman spectroscopy: a new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C. 116, 7515–7519 (2012).
Zhang, R. et al. Photocatalytic oxidative dehydrogenation of ethane using CO2 as a soft oxidant over Pd/TiO2 catalysts to C2H4 and syngas. ACS Catal. 8, 9280–9286 (2018).
Song, H. et al. Visible-light-mediated methane activation for steam methane reforming under mild conditions: a case study of Rh/TiO2 catalysts. ACS Catal. 8, 7556–7565 (2018).
Murata, K. et al. The metal-support interaction concerning the particle size effect of Pd/Al2O3 on methane combustion. Angew. Chem. Int. Ed. 56, 15993–15997 (2017).
Xie, S., Wang, Y., Zhang, Q., Deng, W. & Wang, Y. MgO- and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 4, 3644–3653 (2014).
Ishibashi, K., Fujishima, A., Watanabe, T. & Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2, 207–210 (2000).
Xiao, Q. et al. Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J. Hazard. Mater. 150, 62–67 (2008).
Mu, S. et al. Hydroxyl radicals dominate reoxidation of oxide-derived Cu in electrochemical CO2 reduction. Nat. Commun. 13, 3694 (2022).
Xu, Y. et al. Rate enhancement and rate inhibition of phenol degradation over irradiated anatase and rutile TiO2 on the addition of NaF: new insight into the mechanism. J. Phys. Chem. C. 111, 19024–19032 (2007).
Li, H. et al. Oxygen vacancy associated surface fenton chemistry: surface structure dependent hydroxyl radicals generation and substrate. Environ. Sci. Technol. 51, 5685–5694 (2017).
Zhang, L. et al. Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions. Nat. Energy 7, 1042–1051 (2022).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillonin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Setvin, M. et al. Direct view at excess electrons in TiO2 rutile and anatase. Phys. Rev. Lett. 113, 086402 (2014).
Wang, D., Wang, H. & Hu, P. Identifying the distinct features of geometric structures for hole trapping to generate radicals on rutile TiO2(110) in photooxidation using density functional theory calculations with hybrid functional. Phys. Chem. Chem. Phys. 17, 1549–1555 (2015).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Curtiss, L. A., Raghavachari, K., Redfern, P. C., Rassolov, V. & Pople, J. A. Gaussian-3 (G3) theory for molecules containing first and second-row atoms. J. Chem. Phys. 109, 7764–7776 (1998).
Frisch, M. J. et al. Gaussian 16, Revision C.01 Gaussian Inc. (2016).
Acknowledgements
This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology (No. 2022YFA1504500), the National Natural Science Foundation of China (Nos. 22121001, 22022201, 22132004, 92145301, 92045303, and 2072116), and the Fundamental Research Funds for the Central Universities (No. 20720220008).
Author information
Authors and Affiliations
Contributions
S.X., G.F., and Y.W. conceived and designed the study. H.Z. performed most of the experiments and data analysis. P.S. conducted theoretical calculations and analyzed the data. X.F., Z.H., W.Z., Q.G., and Y.Z. performed characterizations of photocatalysts and photocatalytic processes and analyzed the data. Q.Z. and X.W. provided guidance for experimental design. S.X. guided the experimental work and co-wrote the paper. G.F. guided the computational work and co-wrote the paper. Y.W. supervised the project and co-wrote the paper. All of the authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Venugopala Battula, Zhenfeng Bian, Sebastian Peter and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Sun, P., Fei, X. et al. Unusual facet and co-catalyst effects in TiO2-based photocatalytic coupling of methane. Nat Commun 15, 4453 (2024). https://doi.org/10.1038/s41467-024-48866-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48866-1