Abstract
Electrophilic aromatic substitution is one of the most mechanistically studied reactions in organic chemistry. However, precluded by innate substituent effects, the access to certain substitution patterns remains elusive. While selective C–H alkylation of biorelevant molecules is eagerly awaited, especially for the insertion of a methyl group whose magic effect can boost lead molecules potency, one of the most obvious strategies would rely on electrophilic aromatic substitution. Yet, the historical Friedel-Crafts methylation remains to date poorly selective and limited to activated simple aromatics. Here, we report the development of a selective electrophilic methylation enabling the direct access to highly desirable 1,3-disubstituted arenes. This study demonstrates that this reaction is driven by the generation of long-lived arenium intermediates generated by protonation in superacid and can be applied to a large variety of functionalized (hetero)aromatics going from standard building blocks to active pharmaceutical ingredients.
Similar content being viewed by others
Introduction
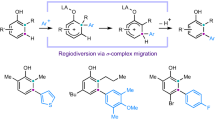
Transformation of aromatic rings is the cornerstone of many large-scale industrial processes with an evident relevance in drug discovery which relies extensively on aromatic scaffolds1. Analysis of benzenoid rings in small molecule active pharmaceutical ingredients (APIs) reveals however a very heterogeneous distribution of substitution patterns (Fig. 1a)2. New synthetic strategies that target underrepresented substitution patterns are therefore eagerly awaited. One of the oldest and most straightforward strategy to substitute an aromatic C–H bond with a functional group is electrophilic aromatic substitution (SEAr)3. However, the level of site-selectivity of a SEAr for a given arene differs widely depending on the nature of the electrophile (Fig. 1b)4. More specifically, it is accepted that the level of para vs. ortho selectivity is mostly dominated by steric effects while the level of para vs. meta selectivity depends on the position of the transition state on the reaction coordinate for the formation of the Wheland intermediates5,6,7. To face this discrepancy, one strategy relies on SEAr methods that allows selective installation of a functional group which can serve as a synthetic handle for further functionalization (e.g., halogenation8, borylation9, TEDAylation10 or thianthrenation11 (Fig. 1c). However, while these approaches improve the level of para-selectivity, the selective access to intrinsically-disfavored positions still remains a highly challenging task12. The meta-selective arylation of phenols via the regiodiversion of an ortho σ-complex to the SEAr-inconsistent meta σ-complex through 1,2-aryl migration was recently reported (Fig. 1d, left)13. Although this method is necessarily limited to the functionalization of ortho-alkylated phenols, it demonstrates that controlling rearrangement of cyclohexadienyl cation intermediates can be synthetically relevant. Such rearrangement is reminiscent of uncontrolled methyl shifts which are known to occur under Friedel–Crafts conditions on toluene derivatives14,15,16. Referred as Baddeley isomerization17 or Friedel–Crafts isomerization14, this reaction has been explored as a piece of exoticism18 but has never been exploited for the regioconvergent methylation of aromatics. Long-lived cyclohexadienyl cations (σ-complexes) can be generated by protonation or alkylation of arenes in weakly-nucleophilic superacid media19,20,21. In an insightful report, McCaulay and Lien have demonstrated that complete isomerization of para-xylene to meta-xylene can be achieved when excess of BF3 Lewis acid is used in combination with HF (Fig. 1d, right)22. Here, the high concentration of H[BF4] superacid allows the full protonation of the arene thereby shifting the equilibrium to the more stable meta-xylene-derived σ-complex. Considering the relevancy to selectively install a methyl group on substituted arenes23,24 and the fact that the Friedel–Crafts methylation25 is arguably one of the least selective SEAr reactions26,27, we designed a superacid-mediated meta-selective electrophilic methylation of arenes based on these conceptual backgrounds.
a Distribution of di-, tri- and tetra-substituted benzenoid substitution patterns in small molecule active pharmaceutical ingredients; 1,2-, 1,4-, and 1,2,4-substituted rings dominate this chemical space2. b Comparison of the regioisomeric distribution of different electrophilic aromatic substitutions of toluene reveals a large discrepancy on regioselectivity depending on the nature of the electrophile4. c Representative examples of the late efforts for the development of para-selective SEAr for the introduction of a synthetic handle. d Conceptual backgrounds: regiodiversion of σ-complexes as an alternative approach toward SEAr-inconsistent benzenoid ring diversification. e Reaction design: our strategy for meta-methylation relies on consecutive Friedel–Crafts type alkylation with a superelectrophilic methylating reagent followed by σ-complex regioconversion through the generation of long-lived arenium ions in superacid.
In this work, we show that under superacid weakly-nucleophilic conditions, after Friedel–Crafts methylation, the resulting product can successively react with superacidic protons to trigger isomerization in favor of the most stable meta-σ-complexes, thus furnishing meta-methylated products after aqueous work-up (Fig. 1e).
Results and discussion
Reaction development
To test our hypothesis, we decided to start our investigations by submitting acetanilide as a model substrate to HF/SbF5 superacid (H0 ≈ –23)28 (Table 1). Using a stoichiometric amount of methyl triflate as alkylating reagent, we observed the formation of a meta/para regioisomers mixture (1m/1p = 57/43) of the expected methylated acetanilide 1 in 61% yield after 2 h at −40 °C (Table 1, entry 1). Gratifyingly, raising the temperature favored the selective formation of the targeted meta product 1m and only traces of 1p was observed after 2 h at 0 °C (Table 1, entries 2 and 3). This is in striking contrast to other SEAr reactions of the same substrate in similar conditions which favor para-functionalization29,30,31,32,33. Screening of a large selection of methylating reagents (full description in SI) eventually allowed us to identify trimethyl phosphate and dimethyl sulfate as convenient electrophilic methyl sources under these conditions, also affording 1m in very good yield (Table 1, entries 4 and 5).
Scope of application
With these optimized conditions in hand, we next explored the scope of application of this transformation (Fig. 2). 2-Haloacetanilides were exclusively methylated at position 4 (2, 3) while 3-bromacetanilide afforded the 1,3,5-trisubstituted derivative 4 albeit in moderate yields due to the deactivating nature of the halogen atoms. SEAr reactions of haloacetanilides under similar conditions usually favor para- or ortho-selectivity with respect to the halo-substituent29,30,31,32,33. In comparison, the reaction of fluorobenzene under the same conditions afforded mostly meta-fluorotoluene (5). This result is noteworthy as superelectrophile-promoted methylation of the same substrate was recently reported to give a 38:11:51 mixture of ortho-, meta- and para-fluorotoluenes25. 3-Methoxy- and 3-alkylacetanilides were converted into their meta-methylated analogues 6–9. The low yield obtained for the methoxy derivative (6) can be explained by the protonation of the alkoxy substituent under these conditions which considerably lowers the nucleophilicity of the protonated substrate in equilibrium with non-protonated 3-methoxyacetanilide. The methylation of phenanthridinone afforded compound 10 in very high yield although the regioselectivity of the reaction was significantly lower in this case probably due to the dissonant directing effect of the amide and aryl substituents. Methylation of dihydroquinolinone and indolinones favored the less hindered meta-position to the amide moiety (products 11–13). The non-innate site selectivity of this transformation is even more striking starting from the 2-methylindole. While indoles are known to be excellent nucleophiles at C3 position, the methylation occurred exclusively on the benzene ring under these conditions affording mostly the C6-methyl derivative 14. This unusual regioselectivity can be attributed to the formation of an iminium ion after protonation of the pyrrole ring in superacid. Next, we probed the compatibility of the transformation with common functional groups installed on the side chain of alkylbenzene derivatives. The reaction proceeded equally well with substrates bearing amide (15–17), cyclic carbamate (18), nitrile (19), ester (20) or ketone (21) functionalities with good to excellent meta-selectivity. Noteworthy, chiral products 18 and 20 were obtained from their enantiopure precursors with no sign of racemization. The reaction of 4-methoxybiphenyl afforded the meta-methylated compound 22 with exclusive functionalization of the phenyl ring, the electron-richer anisyl moiety being most likely protonated under these conditions and thus less reactive toward SEAr. Similarly, biphenyls bearing an electron-withdrawing group reacted selectively at meta position of the less deactivated benzene ring as exemplified with products 23–26. In comparison, acetophenone was fully recovered when subjected to optimized methylation conditions. Phenylpyridines could be efficiently methylated on the phenyl moiety despite the deactivating nature of the pyridyl group (most likely in equilibrium with its protonated form under the reaction conditions) with good to excellent meta-selectivity (27–30). Intermolecular SEAr on phenylpyridines are very scarce in the literature and rare examples of acid-promoted nitration show the preferential functionalization of the para position with low level of selectivity (p/m = 1.2–1.4 for the nitration of 2- and 4-phenylpyridine)34,35. On the other hand, the strongly deactivated 4-phenylpyrimidine did not react, even at room temperature on prolonged reaction time. An important feature is that under these conditions, the electron-richer methylated products react slower than the parent arenes—thereby limiting the formation of overalkylated byproducts—as they must be easily protonated to arenium ions. The dimethylation could not be avoided only with 2-phenylimidazole and 2-phenylthiazole (even with a lower amount of methylating reagent) but the meta-dimethylated products 31 and 32 could be isolated in good yields. In these cases, protonation of the azole moiety presumably disfavors the arenium ion formation by protonation after the first methylation allowing further alkylation. We next focused on the late-stage methylation of substrates derived from natural or synthetic drug-like molecules36. Satisfyingly, the aromatic amides derived from norfentanyl (33), diclofenac (34) and tryptamine (35) were selectively methylated in high yields under optimized conditions. N-Ac-5-chlorotryptamine, precursor of serotonin-like molecules, and the natural alkaloid vinburnine reacted both selectively on the benzene ring of their indole subunit in reasonable yields (36, 37). The reaction was also found very efficient with the benzofuran-derived benzbromarone (38) although the C5/C6 selectivity was somewhat lower in this case. The reaction was also amenable to the methylation of aromatic amino acids as exemplified with phenylglycine- and phenylalanine-derived product 39 and 40, both obtained enantiomerically pure. The preferential dimethylation observed for compound 39 might be explained by the protonation of both acetamide and ester functions which might hamper aromatic protonation by repulsive effect, thereby increasing its propensity to overreact with the alkylating reagent. Similarly, phenylalanine-derived dipeptides were selectively methylated (41, 42). The superacid-mediated methylation was also found very efficient on phenethylamine derivatives with very high level of selectivity as exemplified with the formation of products 43 and 44. Derivatives of the nonsteroidal anti-inflammatory drugs fenbufen (45) and flurbiprofen (46) were methylated with moderate to excellent efficiency on the less-substituted phenyl ring albeit with modest selectivity in the case of 46. Finally, as observed for products 31 and 32, desmethyl celecoxib underwent dimethylation under the optimized conditions to afford a meta-dimethylated analogue of celecoxib 47.
Overall yield and regioselectivity determined by NMR or HPLC analysis of the crude product (isolated yield of pure major regioisomer in parenthesis). Reaction conditions: arene (1 equiv.), Me2SO4 (1 equiv.), HF/SbF5 (v/v = 1/1), 0 °C, 4 h. a After 2 h. b Using Me3PO4 (0.37 equiv.). c At room temperature. d After 16 h. e Using Me3PO4 (1 equiv.). f Isolated as mixture of regioisomers (calculated yield of major regioisomer in parenthesis). g Yield of major regioisomer only; estimated by HPLC analysis of the crude product (isolated yield in parenthesis). h Using (CD3)2SO4 (1 equiv.). i Using (13CH3)2SO4 (1 equiv.).
Importantly, this method is also efficient to install isotopically-labeled methyl groups owing to the ready availability of dimethyl sulfate isotopologues. Since 2017 and the first FDA approval of a CD3-containing drug (deutetrabenazine)37, the development of new methods to introduce a trideuteromethyl group has received a considerable attention38,39. By using either (CD3)2SO4 or (13CH3)2SO4 under the optimized conditions, six selected representative molecules were efficiently methylated with consistent degree of selectivity to afford the corresponding isotopically-labeled products adorned with a CD3 or a 13CH3 group (Fig. 2, bottom). Noteworthy, no sign of hydrogen isotope exchange was detected from the deuterated products.
Nature of the reactive methylating reagents
The original Friedel–Crafts alkylation relies on the polarization of an alkyl halide by complexation of a Lewis acid such as AlCl3 to generate an alkylating species whose carbocationic character depends on the stabilizing effect of its substituents. Formation of free CH3+ is very unlikely and the Friedel–Crafts methylation with MeCl has been suggested to proceed via the formation of a dimethylchloronium cation40,41,42,43. On the other hand, dimethyl sulfate and trimethylphosphate are convenient reagents for heteroatom methylation under neutral or basic conditions but their use as Friedel–Crafts reagents in acidic conditions has never been reported. Therefore, the behavior of dimethyl sulfate and trimethylphosphate in HF/SbF5 solution was explored by low-temperature NMR spectroscopy to gain some information on the nature of the reactive species in superacid (Table 2, see SI for full analysis). The clean 1H NMR spectrum obtained at −50 °C from dimethyl sulfate displays a singlet slightly shielded to 3.11 ppm (compared with a singlet at 3.98 ppm for Me2SO4 in acetone-d6). This can be attributed to O-protonation of dimethyl sulfate although no proton on oxygen could be directly observed due to fast exchange as previously suggested44. Olah et al. reported a δ 1H of 4.85 ppm in the FSO3H/SbF5/SO2ClF superacid system44. The difference of chemical shift with our value might be explained by solvent effect which strongly influences 1H NMR analysis. The 13C NMR spectrum shows a single signal at 66.4 ppm deshielded by 7 ppm compared to that of dimethyl sulfate in acetone-d6 accounting for the strong electron-withdrawing effect induced on the methyl group. Importantly, no sign of the MeF·SbF5 complex45 was detected by 1H, 13C or 19F NMR analysis precluding its role as an active species in this transformation46. No major changes in the 1H and 13C NMR spectra were observed at 0 °C suggesting that the reactive species in the reaction conditions is a protonated form of dimethyl sulfate, although exact structural assignment remains elusive on this sole basis (see below for the proposition from DFT calculations). Similarly, 1H NMR spectrum of a solution of trimethyl phosphate in HF/SbF5 at −50 °C shows a slight shielding of the doublet corresponding to the three methyl groups while the 13C NMR signal is deshielded by 3.3 ppm compared to the signal of the parent molecule in acetone-d6. Moreover, the 31P NMR spectrum displays a signal at 0.81 ppm slightly shielded compared to the signal observed in acetone-d6 (2.30 ppm). All these data are in agreement with a protonation on the phosphoryl oxygen atom (also confirmed by the computed protonation energies and δ 13C, see below) although fast exchange with surrounding excess acid prevents its direct observation by 1H NMR analysis47. 1H, 13C and 31P NMR analysis of this solution at 0 °C reveals the presence of the same species but accompanied with several other organophosphorus compounds which we could not precisely identified. The presence of three sets of signals centered at 0.9, −5.2 and −11.6 ppm in the 31P NMR spectrum could however indicate the formation of polyphosphate derivatives48 over time which might also act as methylating reagents. The case of methyl triflate is slightly more complicated. In superacid, we observed the formation of three species amongst which we could readily identify TfOH (δ 13C: 116.9 ppm, q, J = 319.2 Hz/δ 19F: −76.2 ppm; see SI), most likely under its protonated form CF3SO3H2+49. The remaining two set of signals observed in the 1H, 13C and 19F spectra are very similar and could be attributed to a protonated form of TfOMe and to a dimethylated oxonium salt arising from the disproportionation of TfOMe in superacid (along with TfOH). Both species display a singlet around 3.5 ppm in the 1H NMR spectrum, slightly shielded compared to that of TfOMe in CD2Cl2. The 13C NMR spectrum consists of two deshielded singlets around 71 ppm, both accounting for methyl groups, and two quartets around 117 ppm (J = 321 and 324 Hz) which fit very well with the 13C NMR signature of a trifluoromethylsulfonium salt50. Also, the observed 19F NMR signals (−71.3 and −74.0 ppm) are very close to the signal reported for [CF3SO3(SiMe3)2]+[B(C6F5)4]− in benzene-d6 (δ 19F: −74.1 ppm)50. From this NMR study, we could propose structures for the methylating reagents in superacid (Table 2, bottom). In each case, the preferred site of protonation was further confirmed by DFT calculations at the ωB97xD/def2-TZVP + PCM level of theory (see SI for full detail) also supported by computed 13C NMR shifts (B3LYP/cc-pvTZ//ωB97xD/def2-TZVP). Thus, the main reactive species from dimethylsulfate and trimethylphosphate would consist in a protonated form of these reagents while methyl triflate would generate a mixture of [CF3SO3HMe]+ and [CF3SO3(Me)2]+, both acting as superelectrophilic methylating reagents.
Rationalization of the observed regioselectivity
According to literature and our own experimental observations, we supposed that the non-innate selectivity observed for this electrophilic methylation might come, at least in part, from the rearrangement of methylated products in superacid. Monitoring the methylation of acetanilide under the optimized conditions showed the initial formation of a mixture of 1p and 1m within the first minutes which rapidly evolved in favor of 1m to reach full conversion after 2 h (Fig. 3a). Moreover, when 1p was submitted to HF/SbF5 at 0 °C, it was fully converted into 1m after 1 h thus supporting our hypothesis (Fig. 3b). This rearrangement was much slower at −20 °C and almost inoperative at −40 °C. This indicates that the large proportion of meta-methylated product observed at −40 °C with TfOMe (Table 1, entry 1) does not come from the isomerization of 1p but is rather the consequence of the poor intrinsic selectivity of the Friedel–Crafts methylation. The absence of isomerization with other acid promoters revealed the necessity to use strong superacidic media to promote the rearrangement of acetanilide 1p (Fig. 3b). The generality of this process was then evaluated on four representative examples (Fig. 3c). The para isomers of compounds 19, 23 and 29 and the C5-methylated compound 14 were thus submitted to the optimized conditions affording the corresponding rearranged products with the same degrees of selectivity than those observed from the direct methylation of their parent arenes. Importantly, when pure meta regioisomer 29m was submitted to HF/SbF5, compound 29 was recovered as a meta/para isomer mixture (m/p = 82:18) similar to the one obtained from the rearrangement of 29p, suggesting a thermodynamically-controlled isomerization.
a Monitoring of the Friedel–Crafts methylation of acetanilide (monitored by 1H NMR analysis of aliquot samples of the reaction mixture). b Control experiments for the isomerization of 1p to 1m (monitored by 1H NMR analysis of aliquot samples of the reaction mixtures). c Isomerization of compounds 14, 19, 23, 29 (overall yield of the mixture of regioisomers; regioselectivity determined by NMR analysis of the crude product). d Attempt of intramolecular competition between the methylation of a C–H or a C–D bond after methyl migration from 1p-d. e KIE study through intermolecular competition and by separate experiments between two isotopologues 1p and 1p-d3 (kH/kD average values obtained from three experiments, see SI).
To gain a deeper understanding of the bond cleavage/bond formation event occurring during the methyl shift, we next explored deuterium kinetic isotope effects (KIE). We first set up an intramolecular competition experiment from the partially-labeled compound 1p-d (Fig. 3d). However, this compound readily undergoes full hydrogen isotope exchange even at −40 °C preventing any success in measuring an eventual KIE. Nevertheless, this experiment demonstrates that C3 protonation of 1p under these conditions is a fast and reversible process. KIE study through intermolecular competition and by separate experiments between two isotopologues 1p and 1p-d3 was next examined. No appreciable secondary kinetic isotope effect (SKIE) could be observed from these experiments (Fig. 3e). A positive SKIE would have been expected if a positive charge was developed at C4 during the selectivity-determining step due to hyperconjugative effect51. This absence of normal SKIE also discards a carbocationic character of the methyl group during the selectivity-determining step as a positive α-SKIE would have also been expected if change in hybridization from sp3 to sp2 was taking place52. This contrasts severely with related rate-controlling methyl shifts for which a substantial amount of positive charge is born by the migrating methyl group, decreasing C–H stretching and bending force constants, which is reflected by normal SKIE values (kH/kD = 1.1–1.2)53,54. On the other hand, the small inverse SKIE observed for the Wagner-Meerwein rearrangement of neopentyl brosylate (kH/kD = 0.98) has been interpreted as the consequence of methyl migration occurring after the rate-controlling step53. Accordingly, we propose that the superacid-mediated aromatic methyl shift takes place after a rate-controlling arenium ion formation through C4 protonation.
To explore the transient formation of such arenium ions, we next studied the behavior of methylated arenes in superacid by low-temperature NMR spectroscopy (Fig. 4a). We started our investigations by submitting compound 1p to HF/SbF5 at −40 °C to prevent any isomerization. However, no clean 1H or 13C NMR spectra could be collected from this experiment. Upon prolonged reaction time, signals corresponding to dication 1A started to appear. The structure of 1A was confirmed by submitting pure 1m to HF/SbF5 under similar conditions which gave the same spectrum. This species 1A is characterized by a broad singlet at δ(1H) = 3.56 ppm correlating with δ(13C) = 42.1 ppm in the HSQC spectrum which was attributed to the methylene moiety resulting from aromatic protonation para to the acetamido group. This is also evidenced in the 13C NMR spectrum by the presence of two strongly deshielded signals at 170.2 and 204.3 ppm consistent with the formation of a cyclohexadienyl cation with a delocalized positive charge55. The observation of this arenium ion is particularly noteworthy considering that the acetamide moiety is also protonated as confirmed by the signal at 11.30 ppm in the 1H NMR spectrum. Similar analysis performed on other representative methylated products allowed the characterization of 19A, 23A, 29A and 14A which represent rare examples of long-lived arenium dications in solution56. Collectively, these data suggest that the isomerization is thermodynamically driven by the relative stability of arenium ions under superacidic conditions.
a Left: key NMR signals of cationic intermediates obtained from a representative panel of products in HF/SbF5 at −40 °C using acetone-d6 as external standard. 1H NMR signals in green, 13C NMR signals in blue or red. Right: 1H and 13C NMR spectra of dication 14A as a representative example. b Relative free energies of arenium isomers derived from compounds 1, 19, 23, 29 and control compounds 48 and 49 in their monocationic and dicationic forms computed at the ωB97xD/def2-TZVP + PCM (using the dielectric constant of HF: ε = 83.6 at 273 K) level of theory. Only the lowest energy conformers were considered in each case for the sake of clarity (see SI for full computational details).
To obtain more information on this aspect, we then computed the relative free energies of arenium ions derived from compounds 1, 19, 23 and 29 by DFT calculations at the ωB97xD/def2-TZVP + PCM (HF)57 level of theory (Fig. 4b). For this, we considered four arenium isomers (A–D) in their monocationic and dicationic forms (aromatic functional group R either protonated or not). We furthermore conducted full conformer search using the CREST methodology developed by Grimme et al.58 to ensure the lowest energy conformers were systematically considered (see SI for the full details of the methodology). In the monocationic series, meta-methylated arenium isomers A were found the most stable ones in each case (by at least 2.6 kcal/mol). As anticipated, similar results were obtained from control computational analysis of xylene-derived arenium ions (48). On the other hand, para-methylacetophenone-derived arenium ion 49B was found more stable in comparison with 49A. This can be directly related to the strong electron-withdrawing effect of the ketone functionality which prevents the development of any cationic charge in meta position of the acetyl group. In fact, any tentative of isomerization failed in our hand when para-methylacetophenone 49p was subjected to HF/SbF5 at 0 °C for 2 h. Considering the ability to generate dications in these superacidic conditions, we next studied the influence of the protonation of the aromatic functional groups on the relative stability of arenium ions. The dicationic forms of 1A and 23A were found more stable than 1B and 23B respectively by 2.1 and 1.2 kcal/mol despite the protonation of the amide and ketone functionalities. On the other hand, para-methylated 19B and 29B were found the most stable isomers in their dicationic forms (by 0.7 and 1.2 kcal/mol compared with 19A and 29A). In these cases, the dicationic forms must be in a disfavored equilibrium with their monocationic forms which would mostly dictate the orientation of the σ-complex regioconversion. This is also in accordance with the constant ratio of meta and para isomers of 29 observed after isomerization of either pure 29m or 29p (Fig. 3c). This equilibrium would also account for the lower resolution of 1H and 13C NMR spectra obtained from compounds 19m and 29m in superacid (Fig. 4a).
To conclude, we have developed a direct and selective method for the meta-methylation of arenes. Although inefficient with electron-deficient arenes, this transformation exhibits a broad substrate scope, tolerating synthetically useful functionalities, and is also efficient to functionalize biorelevant molecules at a later stage of a synthetic plan. The weakly nucleophilic superacid solution is crucial for the selectivity of the process that is controlled by the stabilization of σ-complex intermediates generated after methylation/isomerization. Additional kinetic, spectroscopic and computational investigations provide insights into this mechanism. We believe that this study will pave the way for the emergence of new synthetic strategies exploiting long-lived cyclohexadienyl cations.
Methods
General procedure for superacid-mediated Friedel–Crafts methylation
To a solution of HF/SbF5 (v/v 1/1, 1–2 ml) was added aromatic starting material (0.2–0.5 mmol) at 0 °C unless stated otherwise. The methylating reagent (Me2SO4 or Me3PO4 as indicated in Fig. 2) was added and the reaction mixture was stirred at the same temperature for 2–16 h (as indicated in Fig. 2). The reaction mixture was then poured onto an ice-cold saturated aqueous solution of Na2CO3. The aqueous phase was extracted thrice with CH2Cl2. The combined organic layers were dried over MgSO4 and concentrated under reduced pressure. The crude residue was then purified by flash column chromatography over silica gel using the eluent system described for each compound in the Supplementary Information.
Caution
HF/SbF5 is highly toxic and corrosive. Handling of hydrogen fluoride and antimony pentafluoride must be done by experienced chemists with all the necessary safety arrangements in place. Direct exposure must be avoided. Use calcium gluconate gel to treat the affected skin area in case of skin exposure. Reactions performed in superacid were carried out in a screw-capped Teflon® flask with a magnetic stirrer. No further precautions have to be taken to prevent mixture from moisture.
Data availability
All data generated and analyzed during this study, which include experimental, spectroscopic and computational data, are included in this article and its Supplementary Information. The relative free energies data and the Cartesian coordinates are available as a Supplementary dataset. Should any raw data files be needed in another format they are available from the corresponding author upon request.
References
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Nilova, A., Campeau, L.-C., Sherer, E. C. & Sturat, D. R. Analysis of benzenoid substitution patterns in small molecule active pharmaceutical ingredients. J. Med. Chem. 63, 13389–13396 (2020).
Taylor, R. Electrophilic Aromatic Substitution (John Wiley & Sons, Inc. 1990).
Stock, L. M. & Brown, H. C. A quantitative treatment of directive effects in aromatic substitution. Adv. Phys. Org. Chem. 1, 35–154 (1963).
Rys, P., Skrabal, P. & Zollinger, H. Structure and stereochemistry of the transition states and intermediates of heterolytic aromatic substitutions. Angew. Chem. Int. Ed. Engl. 11, 874–883 (1972).
Olah, G. A. Aromatic substitution. XXVIII. Mechanism of electrophilic aromatic substitutions. Acc. Chem. Res. 4, 240–248 (1971).
Juliá, F. et al. High site selectivity in electrophilic aromatic substitutions: mechanism of C–H thianthrenation. J. Am. Chem. Soc. 143, 16041–16054 (2021).
Tang, R.-J., Milcent, T. & Crousse, B. Regioselective halogenation of arenes and heterocycles in hexafluoroisopropanol. J. Org. Chem. 83, 930–938 (2018).
Kim, S. et al. Arene C–H borylation strategy enabled by a non-classical boron cluster-based electrophile. Nat. Commun. 14, 1671 (2023).
Serpier, F. et al. Selective methylation of arenes: a radical C−H functionalization/cross-coupling sequence. Angew. Chem. Int. Ed. 57, 10697–10701 (2018).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Kumar Sinha, S. et al. Toolbox for distal C–H bond functionalizations in organic molecules. Chem. Rev. 122, 5682–5841 (2022).
Senior, A., Ruffell, K. & Ball, L. T. meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution. Nat. Chem. 15, 386–394 (2023).
Olah, G. A. & Meyer, M. W. Friedel-Crafts isomerization. IV. Aluminum halide-catalyzed isomerization of halotoluenes. J. Org. Chem. 27, 3464–3469 (1962).
Norris, J. F. & Vaala, G. T. The rearrangement of the xylenes by aluminum chloride. J. Am. Chem. Soc. 61, 2131–2134 (1939).
Olah, G. A. & Lapierre, J. C. Friedel–Crafts isomerization. XII. Aluminum chloride catalyzed isomerization of the methylbiphenyls. J. Org. Chem. 31, 1271–1272 (1966).
Baddeley, G. & Kenner, J. 64. The meta-alkylation of aromatic hydrocarbons by the Friedel-Crafts reaction. J. Chem. Soc. 303–309 https://doi.org/10.1039/JR9350000303 (1935).
Olah, G. A. Friedel-Crafts Chemistry (Wiley, 1973).
Olah, G. A., Prakash, G. K. S., Molnar, A. & Sommer, J. Superacid Chemistry (Wiley, 2009).
Koptyug, V. A. Arenonium ions (structure and reactivity). Russ. Chem. Bull. 23, 1031–1045 (1974).
MacLean, C., van der Waals, J. H. & Mackor, E. L. Proton magnetic resonance of aromatic carbonium ions. Mol. Phys. 1, 247–256 (1958).
McCaulay, D. A. & Lien, A. P. Isomerization of the methylbenzenes. J. Am. Chem. Soc. 74, 6246–6250 (1952).
Aynetdinova, D. et al. Installing the “magic methyl”—C–H methylation in synthesis. Chem. Soc. Rev. 50, 5517–5563 (2021).
Barreiro, E. J., Kummerle, A. E. & Fraga, C. A. M. The methylation effect in medicinal chemistry. Chem. Rev. 111, 5215–5246 (2011).
He, T., Klare, H. F. T. & Oestreich, M. Catalytically generated Meerwein’s salt-type oxonium ions for Friedel–Crafts C(sp2)–H methylation with methanol. J. Am. Chem. Soc. 145, 3795–3801 (2023).
Olah, G. A., Molnar, A. & Surya Prakash, G. K. Alkylations. in Hydrocarbon Chemistry 3rd edn (eds Olah, G. A., Molnar, A. & Surya Prakash, G. K.) 305–387 (John Wiley & Sons, Inc, 2017).
Dong, P. et al. Progress of methylation of c6−8∼arene with methanol: mechanism, catalysts, kinetic/thermodynamics and perspectives. Processes 10, 881 (2022).
Jost, R. & Sommer, J. Tracking the limits of superacidity. Rev. Chem. Intermed. 9, 171–199 (1988).
Bonazaba Milandou, L. J. C. et al. Superacid-catalyzed trifluoromethylthiolation of aromatic amines. Angew. Chem. Int. Ed. 56, 169–172 (2017).
Mamontov, A. et al. Complementary site-selective halogenation of nitrogen-containing (hetero)aromatics with superacids. Chem. Eur. J. 26, 10411–10416 (2020).
Bourbon, P., Appert, E., Martin-Mingot, A., Michelet, B. & Thibaudeau, S. Complementary site-selective sulfonylation of aromatic amines by superacid activation. Org. Lett. 23, 4115–4120 (2021).
Artault, M., Vitse, K., Martin-Mingot, A. & Thibaudeau, S. Direct superacid-promoted difluoroethylation of aromatics. Chem. Eur. J. 28, e202103926 (2022).
Debarge, S., Violeau, B., Bendaoud, N., Jouannetaud, M.-P. & Jacquesy, J.-C. Regioselective electrophilic trifluoromethylation of substituted anilines and derivatives in superacid. Tetrahedron Lett. 44, 1747–1750 (2003).
Katritzky, A. R. & Kingsland, M. The kinetics and mechanism of the electrophilic substitution of heteroaromatic compounds. Part XIII. The mononitration of 2-phenylpyridine and its N-oxide. J. Chem. Soc. B 862–864 https://doi.org/10.1039/J29680000862 (1968).
De Sarlo, F. & Ridd, J. H. Inductive and field effects in aromatic substitution. Part I. Kinetics of nitration of 4 phenylpyridine and 4 benzylpyridine. J. Chem. Soc. B 712–715 https://doi.org/10.1039/J29710000712 (1971).
Friis, S. D., Johansson, M. J. & Ackermann, L. Cobalt-catalysed C–H methylation for late-stage drug diversification. Nat. Chem. 12, 511–519 (2020).
Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 35, 493–494 (2017).
Steverlynck, J., Sitdikov, R. & Rueping, M. The deuterated “magic methyl” group: a guide to site-selective trideuteromethyl incorporation and labeling by using CD3 reagents. Chem. Eur. J. 27, 11751–11772 (2021).
Concetta Di Martino, R. M., Maxwell, B. D. & Pirali, T. Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug Discov. 22, 562–584 (2023).
Olah, G. A. & DeMember, J. R. Friedel-Crafts chemistry. IV. Dialkylhalonium ions and their possible role in Friedel-Crafts reactions. J. Am. Chem. Soc. 91, 2113–2115 (1969).
Olah, G. A. & DeMember, J. R. Friedel-Crafts chemistry. V. Isolation, carbon-13 nuclear magnetic resonance, and laser Raman spectroscopic study of dimethylhalonium fluoroantimonates. J. Am. Chem. Soc. 92, 718–720 (1970).
Stoyanov, E. S., Stoyanova, I. V., Tham, F. S. & Reed, C. A. Dialkyl chloronium ions. J. Am. Chem. Soc. 132, 4062–4063 (2010).
Hämmerling, S. et al. A very strong methylation agent: [Me2Cl][Al(OTeF5)4]. Angew. Chem. Int. Ed. 58, 9807–9810 (2019).
Olah, G. A., Ku, A. T. & Olah, J. A. Stable carbonium ions. CXVII. Protonation of sulfites and sulfates and their cleavage reactions in fluorosulfuric acid-antimony pentafluoride solution. J. Org. Chem. 35, 3929–3932 (1970).
Olah, G. A., Donovan, D. J. & Lin, H. C. Friedel-Crafts chemistry. 10. Observation of the methyl fluoride-antimony pentafluoride complex in sulfuryl fluoride solution, an exceedingly low nucleophilicity solvent. Reinvestigation of the complex in sulfur dioxide and sulfuryl chloride fluoride solution showing O-methylation. J. Am. Chem. Soc. 98, 2661–2663 (1976).
Olah, G. A., DeMember, J. R. & Schlosberg, R. H. Friedel-Crafts chemistry. III. Methyl fluoride-antimony pentafluoride, a powerful new methylating agent. Methylation reactions and the polycondensation of methyl fluoride. J. Am. Chem. Soc. 91, 2112–2113 (1969).
Olah, G. A. & McFarland, C. W. Organophosphorus compounds. XII. Proton and phosphorus-31 NMR spectroscopic studies of the protonation and cleavage of trialkyl(aryl)phosphates and phosphites, dialkyl phosphonates, and phosphorus oxy acids in fluorosulfuric acid, and fluorosulfuric acid-antimony pentafluoride. J. Org. Chem. 36, 1374–1378 (1971).
Kawabe, M., Ohashi, O. & Yamaguchi, I. Phosphorus nuclear magnetic resonance in polyphosphates and determination of their hydrolysis rate constants. Bull. Chem. Soc. Jpn. 43, 3705–3710 (1970).
Soltner, T., Goetz, N. R. & Kornath, A. The protonation of CF3SO3H: preparation and characterization of trifluoromethyldihydroxyoxosulfonium hexafluoridoantimonate, CF3SO3H2+SbF6–. Eur. J. Inorg. Chem. 20, 3076–3081 (2011).
Schulz, A., Thomas, J. & Villinger, A. Preparation and characterization of [CF3SO3(SiMe3)2]+[B(C6F5)4]−. Chem. Commun. 46, 3696–3698 (2010).
Leffek, K. T., Llewellyn, J. A. & Robertson, R. E. Some deuterium kinetic isotope effects: IV. β-Deuterium effects in water solvolysis of ethyl, isopropyl, and tert-butyl compounds. Can. J. Chem. 38, 2171–2177 (1960).
Streitwieser, A. Jr, Jagow, R. H., Fahey, R. C. & Suzuki, S. Kinetic isotope effects in the acetolyses of deuterated cyclopentyl tosylates. J. Am. Chem. Soc. 80, 2326–2332 (1958).
Schubert, W. M. & LeFevre, P. H. Kinetic isotope effects and CD3 vs. CH3 migration. J. Am. Chem. Soc. 91, 7746–7748 (1969).
Vitullo, V. P. & Logue, E. A. Cyclohexadienyl cations. 6. Methyl group isotope effects in the dienone-phenol rearrangement. J. Am. Chem. Soc. 98, 5906–5909 (1976).
Reed, C. A. et al. Isolating benzenium ion salts. J. Am. Chem. Soc. 125, 1796–1804 (2003).
Prakash, G. K. S., Rawdah, T. N. & Olah, G. A. Stable carbodications. Angew. Chem. Int. Ed. Engl. 22, 390–401 (1983).
Cole, R. H. Dielectric constant and association in liquid HF. J. Chem. Phys. 59, 1545–1546 (1973).
Pracht, P., Bohle, F. & Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 22, 7169–7192 (2020).
Acknowledgements
We gratefully acknowledge the Région Nouvelle-Aquitaine for financial support (IsoTop—Allocation no. APE01152, Ph.D. grant to P.B.). We also acknowledge the University of Poitiers, the Centre National de la Recherche Scientifique (CNRS), the European Union (ERDF), @rtMolecule society, ACTIV-H Company, and the French Fluorine Network (GIS-FLUOR).
Author information
Authors and Affiliations
Contributions
Conceptualization: B.M. and S.T.; Methodology: B.M. and S.T.; Investigation: P.B., K.V., A.M.M., H.G., F.G. and B.M.; Writing: B.M. and S.T., Review & editing: B.M. and S.T.; Funding acquisition: S.T.; Supervision: A.M.M., B.M. and S.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Lutz Greb, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bourbon, P., Vitse, K., Martin-Mingot, A. et al. Leveraging long-lived arenium ions in superacid for meta-selective methylation. Nat Commun 15, 7435 (2024). https://doi.org/10.1038/s41467-024-49421-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-49421-8