Abstract
A strong and confined Brønsted acid catalyzed enantioselective cyclization of bis(methallyl)silanes provides enantioenriched Si-stereogenic silacycles. High enantioselectivities of up to 96.5:3.5 er were obtained for a range of bis(methallyl)silanes. NMR and ESI-MS studies reveal that the formation of a covalent adduct irreversibly inhibits turnover. Remarkably, we found that acetic acid as an additive promotes the collapse of this adduct, enabling full turnover. Experimental investigation and density functional theory (DFT) calculations were conducted to elucidate the origin of this phenomenon and the observed enantioselectivity.
Similar content being viewed by others
Introduction
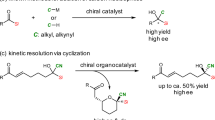
Organosilanes are valuable substances in medicinal chemistry as well as in chemical synthesis and material science1,2,3,4,5. Silicon has several similarities with carbon (e.g., tetrahedral geometry and valency of four), but there are also distinct differences between the two elements (e.g., covalent radius, electronegativity and liphophilicity) making silicon an interesting (bio)isostere of carbon4,5. The synthesis of organosilicon compounds provides new opportunities to drug discovery and scent design. Among the diverse organosilicon compounds, Si-stereogenic silanes have recently demonstrated promising biological activities6,7. For example, Tomooka’s group has shown that one Si-stereogenic silacyclopentane epimer displayed significant binding to the serotonin receptor whereas the opposite epimer showed no binding (Fig. 1a)7. These results underline the importance of silicon stereogenecity in biologically active molecules. However, methods for the preparation of organosilicon compounds possessing a Si-stereogenic center are much less developed as compared to carbon stereogenic molecules.
Even though advances have been made toward the synthesis of Si-stereogenic silanes over the past several decades, many of the reported syntheses have relied on stoichiometric chiral reagents or auxiliaries8,9,10,11,12,13,14,15. More recently, catalytic asymmetric variants including transition-metal catalyzed hydrosilylation of multiple bonds16,17,18,19,20,21,22,23,24,25, alcoholysis of dihydrosilanes26,27,28, Si−H bond insertion of carbenes29,30, Si−C bond cleavage31,32,33,34,35, C–H bond activation36,37,38,39,40,41,42,43 and others44,45,46,47,48,49,50,51 have been disclosed, but they have mostly depended on precious transition-metal catalysts, such as rhodium and palladium52,53,54,55,56. While several enzymatic methods have also been developed57,58, organocatalytic methods only recently emerged59,60,61,62,63.
We recently reported a strongly acidic and confined imidodiphosphorimidate (IDPi) catalyzed synthesis of Si-stereogenic silyl ethers via desymmetrization of bis(methallyl)silanes (Fig. 1b)61,64,65. We also reported a dynamic kinetic asymmetric transformation (DYKAT) of racemic methallyl silanes62. During our previous desymmetrization study, we found that when 2,6-dimethylphenol was excluded, the corresponding silacycle was formed, albeit with poor yield and enantioselectivity (Fig. 1b). Based on this result, we speculated if we could develop the process to access enantioenriched Si-stereogenic silacycles. As the desired reaction product would still be an allylsilane, which is known to be activated by strong acids66,67, a key challenge could be to identify a catalyst system that prevents further activation of the product. Herein, we report an IDPi catalyzed enantioselective cationic cyclization of bis(methallyl)silanes that provides access to Si-stereogenic silacycles (Fig. 1c). Critical to this discovery has been the identification of acetic acid as a catalyst turnover enabling additive. During the preparation of this manuscript, Yu and co-workers reported a chiral imidazolidinone catalyzed intramolecular aldolization of siladials to generate enal-containing Si-stereogenic silacycles (Fig. 1d)63.
Results
Initially, a series of chiral Brønsted acid catalysts were tested for the reaction of bis(methallyl)silane 1a in toluene at −60 °C (Table 1). While chiral phosphoric acid (CPA) 3, disulfonimide (DSI) 468,69 and imidodiphosphate (IDP) 570 showed no reactivity, strongly acidic IDPi 6a gave the desired product albeit in disappointingly low conversion and enantioselectivity (entries 1–4). We speculated the lower conversion of the cyclization reaction compared to our previous intermolecular reaction to be due to the formation of a covalent adduct. Accordingly, we expected that an achiral Brønsted acid additive could accelerate the reaction by efficiently liberating the free catalyst from the adduct. Indeed, phenol, benzoic acid and pivalic acid slightly enhanced the reactivities without change in enantioselectivities (entries 5–7). Acetic acid performed better than other screened additives (entry 8). With acetic acid, we further screened a range of IDPi catalysts. IDPi 6b possessing 4-(tert-butyl)phenyl substituents at 3,3’-positions of the BINOL backbone slightly increased both reactivity and enantioselectivity (entry 9). Modifying the inner core of the IDPi from trifluoromethyl to perfluoronaphthalene-2-yl considerably improved the enantioselectivity as well as reactivity (entries 9–11). By lowering the reaction temperature to −100 °C, the enantioselectivity was further enhanced, albeit with modest conversion. The reactivity could be improved by increasing the amount of acetic acid (entries 12–14). In the absence of acetic acid, the reaction resulted in a disappointing conversion, indicating that an additive is necessary for the reaction (entry 15). Finally, slightly higher catalyst loading (5 mol%) led to full conversion with high enantioselectivity (entry 16). The absolute configuration of the silacycle 2a was determined by Mosher ester analysis after derivatization (see the Supplementary Information for details)71,72.
With optimized conditions in hand, we next examined the substrate scope of the cyclization (Fig. 2). In general, reactions of bis(methallyl)silanes 1a–1g with various alkyl substituents proceed to furnish the silacycle products in good yields and high enantioselectivities. The enantioselectivity decreased slightly as the steric hindrance of alkyl substituent increases from linear alkyl (1a−1e, 93:7 to 95:5 er), isopentyl (1f, 92:8 er) and isobutyl (1g, 91:9 er). The reaction of 2-naphthyl substituted silane 1h afforded the desired products in good yield and high enantioselectivity. While substrate 1i with o-methyl substituent yielded the product with drastically diminished enantioselectivity, substrates 1j and 1k with m- and p-methyl substituents exhibited high enantioselectivities. p-Methoxy and fluoro substituted silanes 1l and 1m were reactive, but slightly reduced enantioselectivities were observed. Of note, bis(methallyl)allylsilane 1n and tris(methallyl)silane 1o were feasible substrates for the reaction, leading to the corresponding silacycle products with high enantioselectivities. Finally, we found that cyclohexyl substituted silane 1p showed low enantioselectivity compared to other silanes.
aReactions were carried out with 0.1‒0.2 mmol of substrate 1, catalyst 6d (5 mol %), acetic acid (1 equiv.) in toluene at the specified temperature for the specified time. Yields are for the isolated compounds. The enantiomeric ratios (er) were determined by HPLC analysis. bPivalic acid was used instead of acetic acid.
To gain mechanistic insight, we conducted several control experiments (Fig. 3). When enantioenriched silacycle 2a was subjected to the reaction conditions in the presence of Tf-substituted catalyst 6b which is more acidic than the corresponding perfluoronaphthyl-2-sulfonyl-substituted catalyst 6d, silyl acetate 7 was formed as major product. In contrast, catalyst 6d did not lead to side product 7, indirectly suggesting that the stability of the silacycle under the reaction conditions depends on the acidity of the IDPi catalysts (Fig. 3a). However, despite the presence of less acidic catalyst 6d, silacycle 2a was completely transformed into side product 7 at room temperature, illustrating the temperature dependence of the silacycle stability (Fig. 3b). As expected, the less reactive bis(allyl)silane 1q did not react under our reaction conditions (Fig. 3c).
To understand the role of the acetic acid additive, we conducted 31P NMR spectroscopic studies with substrate 1a and catalyst 6c (Fig. 4). When 10 equiv. of silane 1a was subjected to catalyst 6c in toluene-d8 solution, eight peaks were immediately observed in the 31P NMR spectrum. Most likely, these peaks originated from in-situ generated covalent adduct 8 and Electrospray Ionization Mass Spectrometry (ESI-MS) analysis further supported the formation of this species73. Free catalyst 6c was also observed with a significant downfield shift of phosphorous signal. Subsequent addition of 10 equiv. of acetic acid to the mixture led to complete regeneration of free catalyst 6c and production of silyl acetate 7. While this reaction reduces the overall yield of the desired product, it enables catalyst turnover.
Based on the control experiments and our NMR and MS studies, a catalytic cycle can be suggested (Fig. 5a). Protonation of the olefin provides ion-pair I consisting of a β-silicon stabilized carbocation and the IDPi anion. Cation-π cyclization of I leads to the formation of ion-pair II74,75,76. Intra ion pair proton transfer within II furnishes the Si-stereogenic silacycle 2, regenerating free catalyst 6. In parallel, ion-pair II can collapse to form covalent adduct 8 which is deleterious to the catalyst turnover. However, acetic acid liberates catalyst 6 from adduct 8, enabling catalyst turnover.
a Proposed catalytic cycle. b Free energy profile of the catalytic cycle calculated at CPCM(Toluene)-ωB97M-V/(ma)-def2-TZVPP//r2SCAN-3c level of theory. The thermal corrections were calculated at 173.15 K. The energy profile leading to the major enantiomer is depicted in black, and the one to the minor enantiomer is in red. The calculated yield and enantiomeric ratio are given by the ratio between 2, 2’, and 8 + 8’ based on the Boltzmann distribution (see the Supplementary Information for details). c Visualized structure of TS4 leading to the covalent adduct 8. Substrate is depicted in magenta.
To gain further insights into the detailed reaction mechanism and investigate the origin of the observed enantioselectivity, we conducted a computational study with substrate 1a and catalyst 6d (see the Supplementary Information for details). Calculation was performed at CPCM(Toluene)-ωB97M-V/(ma)-def2-TZVPP//r2SCAN-3c level of theory to illustrate the relatively small energy difference between two enantiomers77,78,79. Protonation via TS1 is suggested to be the enantiodetermining step of the catalytic cycle, as the subsequent C−C bond forming step is deemed facile. However, the energy difference between TS1 and TS1’, which lead to enantiomers I and I’, is only 0.2 kcal/mol, suggesting that no significant enantioselectivity can be induced in this step (Fig. 5b). After careful analysis, we realized that deprotonation via TS3 and covalent adduct formation via TS4 are key to the observed high enantioselectivity. In the case of the major enantiomer, the deprotonation (TS3, −5.6 kcal/mol) is favored over the covalent adduct formation (TS4, −5.0 kcal/mol). In contrast, for the minor enantiomer, the latter (TS4’, −6.2 kcal/mol) is favored over the former (TS3’, −5.4 kcal/mol). Hence, it is likely that an enantioenrichment process via a parallel kinetic resolution (PKR) takes place at this stage80,81. The major factor here can be attributed to the instability of TS4 compared to TS4’, presumably due to steric hindrance induced by the substrate in TS4 (Fig. 5c). Assuming that the transformation is clean enough to provide only the desired products (2 and 2’) and the adduct 7 (via 8 and 8’), calculating the ratio between them based on the Boltzmann distribution provided the expected yield of the product as 59% and the calculated enantiomeric ratio as 95:5, which are in good agreement with the experimental results (2a, 65% yield, 95:5 e.r.) (see Supplementary Information for details). The proposed mechanism is also supported by the high enantioselectivity of product 2o as the initial protonation intermediate is still achiral. Additionally, the energy of the covalent adduct 8 is found to be −21.4 kcal/mol (8) and −20.9 kcal/mol (8’), significantly lower than the intermediates in the catalytic cycle, suggesting that it cannot return to the catalytic cycle without an additive under the reaction conditions. Therefore, the addition of acetic acid is crucial to achieve a high turnover in this transformation.
Discussion
In summary, we have developed a strong and confined Brønsted acid catalyzed cyclization of bis(methallyl)silanes. A range of bis(methallyl)silanes were converted to the corresponding Si-stereogenic silacycles in a highly enantioselective manner. Mechanistic studies suggest formation of a covalent adduct limiting catalyst turnover. Addition of acetic acid facilitates the rapid regeneration of the catalyst from the covalent adduct, enabling turnover. Furthermore, computational studies suggest the protonation as the enantiodetermining step and the subsequent enantioenrichment as a key to achieve high enantioselectivity. Efforts to develop other organocatalytic methods for a range of Si-stereogenic silanes are currently underway.
Methods
General procedure for the enantioselective cyclization of bis(methallyl)silanes
A GC vial was charged with catalyst 6d (5 mol %), toluene and acetic acid (1 equiv.), and the resulting mixture was cooled to −100 °C in a cryostat. After 10 min, bis(methallyl)silane 1 (0.1 or 0.2 mmol) was slowly added and the GC vial was stored for the given reaction time at the same temperature. After complete conversion indicated by TLC, the reaction was quenched with trimethylamine. The solvent was removed in vacuo and the mixture was purified by column chromatography on silica gel to afford the desired silacycle 2.
Data availability
The experimental procedures and analytical data supporting the findings of this study are available within the manuscript and its Supplementary Information files. Raw and unprocessed NMR data are available from the corresponding author upon request. Cartesian coordinates are available in Supplementary Data 1. Source data are provided with this paper.
References
Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry (Wiley, 2000).
Auner, N. & Weis, J. Organosilicon Chemistry lll: From Molecules to Materials (Wiley, 2009).
Hiyama, T. & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH, 2019).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Ramesh, R. & Reddy, D. S. Quest for novel chemical entities through incorporation of silicon in drug scaffolds. J. Med. Chem. 61, 3779–3798 (2018).
Tacke, R. et al. Biological recognition of enantiomeric silanes: syntheses and antimuscarinic properties of optically active (2-aminoethyl)cyclohexyl(hydroxymethyl)phenylsilanes and related quaternary ammonium derivatives. Organometallics 14, 251–262 (1995).
Igawa, K., Yoshihiro, D., Abe, Y. & Tomooka, K. Enantioselective synthesis of silacyclopentanes. Angew. Chem. Int. Ed. 55, 5814–5818 (2016).
Xu, L.-W., Li, L., Lai, G.-Q. & Jiang, J.-X. The recent synthesis and application of silicon-stereogenic silanes: a renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 40, 1777–1790 (2011).
Bauer, J. O. & Strohmann, C. Recent progress in asymmetric synthesis and application of difunctionalized silicon-stereogenic silanes. Eur. J. Inorg. Chem. 2016, 2868–2881 (2016).
Oestreich, M. Silicon-stereogenic silanes in asymmetric catalysis. Synlett 2007, 1629–1643 (2007).
Rendler, S., Auer, G., Keller, M. & Oestreich, M. Preparation of a privileged silicon-stereogenic silane: Classical versus Kinetic Resolution. Adv. Synth. Catal. 348, 1171–1182 (2006).
Igawa, K., Takada, J., Shimono, T. & Tomooka, K. Enantioselective synthesis of silanol. J. Am. Chem. Soc. 130, 16132–16133 (2008).
Igawa, K., Kokan, N. & Tomooka, K. Asymmetric synthesis of chiral silacarboxylic acids and their ester derivatives. Angew. Chem. Int. Ed. 49, 728–731 (2010).
Bauer, J. O. & Strohmann, C. From an α-functionalized silicon-stereogenic N,O-silane to a monomeric and tetracoordinate tBuLi adduct with lithium-centered chirality. Angew. Chem. Int. Ed. 53, 8167–8171 (2014).
Bai, X.-F. et al. Lewis-base-mediated diastereoselective silylations of alcohols: synthesis of silicon-stereogenic dialkoxysilanes controlled by chiral aryl BINMOLs. Chem. Asian J. 12, 1730–1734 (2017).
Corriu, R. J. P. & Moreau, J. J. E. Asymemtric hydrosilylation of ketones catalysed by a chiral rhodium complex. J. Organomet. Chem. 64, 51–54 (1974).
Hayashi, T., Yamamoto, K. & Kumada, M. Asymmetric synthesis of bifunctional organosilicon compounds via hydrosilylation. Tetrahedron Lett. 15, 331–334 (1974).
Tamao, K., Nakamura, K., Ishii, H., Yamaguchi, S. & Shiro, M. Axially chiral spirosilanes via catalytic asymmetric intramolecular hydrosilylation. J. Am. Chem. Soc. 118, 12469–12470 (1996).
Igawa, K., Yoshihiro, D., Ichikawa, N., Kokan, N. & Tomooka, K. Catalytic enantioselective synthesis of alkenylhydrosilanes. Angew. Chem. Int. Ed. 51, 12745–12748 (2012).
Zhan, G. et al. Enantioselective construction of silicon-stereogenic silanes by scandium-catalyzed intermolecular alkene hydrosilylation. Angew. Chem. Int. Ed. 57, 12342–12346 (2018).
Wen, H., Wan, X. & Huang, Z. Asymmetric synthesis of silicon-stereogenic vinylhydrosilanes by cobalt-catalyzed regio- and enantioselective alkyne hydrosilylation with dihydrosilanes. Angew. Chem. Int. Ed. 57, 6319–6323 (2018).
Lu, W., Zhao, Y. & Meng, F. Cobalt-catalyzed sequential site- and stereoselective hydrosilylation of 1,3- and 1,4-Enynes. J. Am. Chem. Soc. 144, 5233–5240 (2022).
Wang, L., Lu, W., Zhang, J., Chong, Q. & Meng, F. Cobalt-catalyzed regio-, diastereo- and enantioselective intermolecular hydrosilylation of 1,3-Dienes with prochiral silanes. Angew. Chem. Int. Ed. 61, e202205624 (2022).
Huang, Y.-H. et al. Enantioelective synthesis of silicon-stereogenic mohohydrosilanes by rhodium-catalyzed intramolecular hydrosilylation. Angew. Chem. Int. Ed. 61, e202113052 (2022).
Zeng, Y. et al. Rhodium-catalyzed dynamic kinetic asymmetric hydrosilylation to access silicon-stereogenic center. Angew. Chem. Int. Ed. 61, e202214147 (2022).
Corriu, R. J. P. & Moreau, J. E. E. Asymmetric synthesis of alkoxysilanes catalyzed by rhodium complexes. Tetrahedron Lett. 14, 4469–4472 (1973).
Schmidt, D. R., O’Malley, S. J. & Leighton, J. L. Catalytic asymmetric silane synthesis: practical access to chiral silanes. J. Am. Chem. Soc. 125, 1190–1191 (2003).
Zhu, J., Chen, S. & He, C. Catalytic enantioselective dehydrogenative Si–O coupling to access to chiroptical silicon-stereogenic siloxanes and alkoxysilanes. J. Am. Chem. Soc. 143, 5301–5307 (2021).
Yasutomi, Y., Suematsu, H. & Katsuki, T. Iridium(III)-catalyzed enantioselective Si–H bond insertion and formation of an enantioenriched silicon center. J. Am. Chem. Soc. 132, 4510–4511 (2010).
Jagannathan, J. R., Fettinger, J. C., Shaw, J. T. & Franz, A. K. Enantioselective Si–H insertion reactions of diarylcarbenes for the synthesis of silicon-stereogenic silanes. J. Am. Chem. Soc. 142, 11674–11679 (2020).
Shintani, R., Moriya, K. & Hayashi, T. Palladium-catalyzed enantioselective desymmetrization of silacyclobutanes: construction of silacylces possessing a tetraorganosilicon stereocenter. J. Am. Chem. Soc. 133, 16440–16443 (2011).
Zhang, Q.-W. et al. Construction of chiral tetraorganosilicon by tandem desymmetrization of silacyclobutane/intermolecular dehydrogenative silylation. Angew. Chem. Int. Ed. 56, 1125–1129 (2017).
Chen, H. et al. Rhodium-catalyzed reaction of silacyclobutanes with unactivated alkynes to afford silacyclohexenes. Angew. Chem. Int. Ed. 58, 4695–4699 (2019).
Zhang, J., Yan, N., Ju, C.-W. & Zhao, D. Nickel(0)-catalyzed asymmetric ring expansion toward enanitoenriched silicon-stereogenic benzosiloles. Angew. Chem. Int. Ed. 60, 25723–25728 (2021).
Wang, X.-C., Li, B., Ju, C.-W. & Zhao, D. Nickel(0)-catalyzed divergent reactions of silacyclobutanes with internal alkynes. Nat. Commun. 13, 3392–3402 (2022).
Shintani, R., Otomo, H., Ota, K. & Hayashi, T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enanitoselective C–H bond functionalization. J. Am. Chem. Soc. 134, 7305–7308 (2012).
Yang, B., Yang, W., Guo, Y., You, L. & He, C. Enantioselective silylation of aliphatic C–H bonds for the synthesis of silicon-stereogenic dihydrobenzosiloles. Angew. Chem. Int. Ed. 59, 22217–22222 (2020).
Mu, D. et al. Streamlined construction of silicon-stereogenic silanes by tandem enantioselective C–H Silylation/Alkene Hydrosilylation. J. Am. Chem. Soc. 142, 13495–13468 (2020).
Ma, W., Liu, L.-C., An, K., He, T. & He, W. Rhodium-catalyzed synthesis of chiral monohydrosilanes by intramolecular C–H functionalization of dihydrosilanes. Angew. Chem. Int. Ed. 60, 4245–4251 (2021).
Guo, Y., Liu, M.-M., Zhu, X., Zhu, L. & He, C. Catalytic asymmetric synthesis of silicon-stereogenic dihydrodibenzosilines: silicon center-to-axial chirality relay. Angew. Chem. Int. Ed. 60, 13887–13891 (2021).
Zhang, H. & Zhao, D. Synthesis of silicon-stereogenic silanols involving iridium-catalyzed enantioselective C–H silylation of leading to a new ligand scaffold. ACS Catal. 11, 10748–10753 (2021).
Chen, S. et al. Enantioselective construction of six- and seven-membered triorgano-substituted silicon-stereogenic heterocycles. Nat. Commun. 12, 1249–1257 (2021).
Chen, S., Zhu, J., Ke, J., Li, Y. & He, C. Enantioselective intermolecular C–H silylation of heteroarenes for the synthesis of acyclic Si-stereogenic silanes. Angew. Chem. Int. Ed. 61, e202117820 (2022).
Shintani, R., Maciver, E. E., Tamakuni, F. & Hayashi, T. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzooxasilines via enantioselective transmetalation. J. Am. Chem. Soc. 134, 16955–16958 (2012).
Shintani, R., Takagi, C., Ito, T., Naito, M. & Nozaki, K. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles by enantioselective [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 54, 1616–1620 (2015).
Sato, Y., Takagi, C., Shintani, R. & Nozaki, K. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic 5,10-dihydrophenazasilines via enantioselective 1,5-palladium migration. Angew. Chem. Int. Ed. 56, 9211–9216 (2017).
Zhang, G. et al. Asymmetric synthesis of silicon-stereogenic silanes by copper-catalyzed desymmetrizing protoboration of vinylsilanes. Angew. Chem. Int. Ed. 59, 11927–11931 (2020).
Li, S.-S., Sun, S. & Wang, J. Catalytic asymmetric homologation of 4-substituted cyclohexanones with CF3CHN2: enantioselective synthesis of α-trifluoromethyl cycloheptanones. Angew. Chem. Int. Ed. 61, e202115098 (2022).
Gao, J. et al. Copper-catalyzed desymmetrization of prochiral silanediols to silicon-stereogenic silanols. ACS Catal. 12, 8476–8483 (2022).
Yuan, W., Zhu, X., Xu, Y. & He, C. Synthesis of Si-stereogenic silanols by catalytic asymmetric hydrolytic oxidation. Angew. Chem. Int. Ed. 61, e202204912 (2022).
Yang, W. et al. Enantioselective hydroxylation of dihydrosilanes to Si-chiral silanols catalyzed by in situ generated copper(II) species. Angew. Chem. Int. Ed. 61, e202205743 (2022).
Xu, L.-W. Desymmetrization catalyzed by transition-metal complexes: enantioselective formation of silicon-stereogenic silanes. Angew. Chem. Int. Ed. 51, 12932–12934 (2012).
Shintani, R. Recent progress in catalytic enantioselective desymmetrization of prochiral organosilanes for the synthesis of silicon-stereogenic compounds. Synlett 29, 388–396 (2018).
Wu, Y. & Wang, P. Silicon-stereogenic monohydrosilane: synthesis and applications. Angew. Chem. Int. Ed. 61, e202205382 (2022).
Yuan, W. & He, C. Enantioselective C–H functionalization toward silicon-stereogenic silanes. Synthesis 54, 1939–1950 (2022).
Huang, W.-S., Wang, Q., Yang, H. & Xu, L.-W. State-of-the-art advances in enantioselective transition-metal-mediated reactions of silacyclobutanes. Synthesis 54, 5400–5408 (2022).
Djerourou, A.-H. & Blanco, L. Synthesis of optically 2-sila-1,3-propanediols derivatives by enzymatic transesterification. Tetrahedron 32, 6325–6326 (1991).
Lu, X. et al. Catalytic synthesis of functional silicon-stereogenic silanes through candida antarctica lipase B catalyzed remote desymmetrization of silicon-centered diols. Eur. J. Org. Chem. 2013, 5814–5819 (2013).
Liu, H. et al. Stereoselective access to silicon-stereogenic silacycles via the carbene-catalyzed desymmetric benzoin reaction of siladials. ACS Catal. 12, 9864–9871 (2022).
Zhou, M. et al. Construction of tetrasubstituted silicon-stereogenic silanes via conformational isomerization and N-heterocyclic carbene-catalyzed desymmetrization. ACS Catal. 12, 7781–7788 (2022).
Zhou, H. et al. Organocatalytic asymmetric synthesis of Si-stereogenic silyl ethers. J. Am. Chem. Soc. 144, 10156–10161 (2022).
Zhou, H. et al. Organocatalytic DYKAT of Si-stereogenic silanes. J. Am. Chem. Soc. 145, 4994–5000 (2023).
Zhang, X.-X., Yang, G., Zhang, Y.-X., Zhou, J. & Yu, J.-S. Highly enantioselective construction of multifunctional silicon-stereogenic silacycles by asymmetric enamine catalysis. Angew. Chem. Int. Ed. 62, e202217724 (2023).
Kaib, P. S. J., Schreyer, L., Lee, S., Properzi, R. & List, B. Extremely active organocatalysts enable a highly enantioselective addition of allyltrimethylsilane to aldehyde. Angew. Chem. Int. Ed. 55, 13200–13203 (2016).
Schreyer, L., Properzi, R. & List, B. IDPi catalysis. Angew. Chem. Int. Ed. 58, 12761–12777 (2019).
Olah, G. A., Husain, A., Gupta, B. G. B., Salem, G. F. & Narang, S. C. Synthetic methods and reactions 104. silylations with in situ generated trimethylsilyltriflate reagent systems. J. Org. Chem. 46, 5212–5214 (1981).
Morita, T., Okamoto, Y. & Sakurai, H. A simple and efficient preparation of silyl esters and ethers using allyltrimethylsilane/trifluoromethanesulfonic acid. Synthesis 1981, 745–746 (1981).
García-García, P., Lay, F., García-García, P., Rabalakos, C. & List, B. A powerful chiral counteranion motif for asymmetric catalysis. Angew. Chem. Int. Ed. 48, 4363–4366 (2009).
James, T., van Gemmeren, M. & List, B. Development and applications of disulfonimides in enantioselective organocatalysis. Chem. Rev. 115, 9388–9409 (2015).
Čorić, I. & List, B. Asymmetric spirocyclization catalyzed by confined Brønsted acids. Nature 483, 315–139 (2012).
Hoye, T. R., Jeffrey, C. S. & Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2, 2451–2458 (2007).
Allen, D. A., Tomaso, A. E. Jr., Priest, O. P., Hindson, D. F. & Hurlburt, J. L. Mosher amides: determining the absolute stereochemistry of optically-active amines. J. Chem. Educ. 85, 698–700 (2008).
Liu, L. et al. Confined acid-catalyzed asymmetric carbonyl-ene cyclization. J. Am. Chem. Soc. 137, 13268–13271 (2015).
Ishihara, K., Nakamura, S. & Yamamoto, H. The first enantioselective biomimetic cyclization of polyprenoids. J. Am. Chem. Soc. 121, 4906–4907 (1999).
Nakamura, S., Ishihara, K. & Yamamoto, H. Enantioselective biomimetic cyclization of isoprenoids using Lewis acid-assisted chiral Brønsted acids: abnormal Claisen rearrangement and successive cyclizations. J. Am. Chem. Soc. 122, 8131–8140 (2000).
Ishihara, K., Ishibashi, H. & Yamamoto, H. Enantio- and diastereoselective stepwise cyclization of polyprenoids induced by chiral and achiral LBAs. A new entry to (-)-ambrox, (+)-podocarpa-8,11,13-triene diterpenoids, and (-)-tetracyclic polyprenoid of sedimentary origin. J. Am. Chem. Soc. 124, 3647–3655 (2002).
Mardirossian, N. & Head-Gordon, M. J. ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. Chem. Phys. 144, 214110 (2016).
Grimme, S., Hansen, A., Ehlert, S. & Mewes, J.-M. r2SCAN-3c: A “swiss army knife” composite electronic-structure method. J. Chem. Phys. 154, 064103 (2021).
Bursch, M., Mewes, J.-M., Hansen, A. & Grimme, S. Best-practice DFT protocols for basic molecular computational chemistry. Angew. Chem. Int. Ed. 61, e202205735 (2022).
Eames, J. Parallel kinetic resolution. Angew. Chem. Int. Ed. 39, 885–888 (2000).
Dehli, J. R. & Gotor, V. Parallel kinetic resolution of racemic mixtures: a new strategy for the preparation of enantiopure compounds? Chem. Soc. Rev. 31, 365–370 (2002).
Acknowledgements
Generous support was received from the Max Planck Society, the Deutsche Forschungsgemeinschaft (Germany Research Foundation), the Leibniz Award (to B.L.), Germany’s Excellence Strategy (Grant EXC 2033-390677874-RESOLV) and the European Research Council (Early-stage organocatalysis; to B.L.). This work was also financially supported by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, JAPAN by List Sustainable Digital Transformation Catalyst Collaboration Research Platform offered by Hokkaido University, and by JSPS KAKENHI Grants 21H01925 and 20K22515. Part of the computation was performed using Research Center for Computational Science, Okazaki, Japan (Projects: 21-IMS-C303, 22-IMS-C129, and 23-IMS-C119). We also thank the technicians of our group and the members of our NMR, MS and chromatography groups for their excellent service.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
B.L. oversaw the project. J.T.H. developed the reaction and investigated the substrate scope, and conducted the mechanistic studies. J.T.H and M.L implemented NMR studies. N.T. performed the computational studies. Z.H. performed the computational studies on circular dichroism (CD). J.T.H. and B.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): a patent on the general catalyst class and its use in synthesis has been filed.
Peer review
Peer review information
Nature Communications thanks Li-Wen Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, J.T., Tsuji, N., Zhou, H. et al. Organocatalytic asymmetric synthesis of Si-stereogenic silacycles. Nat Commun 15, 5846 (2024). https://doi.org/10.1038/s41467-024-49988-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-49988-2
This article is cited by
-
Phase-transfer-catalyst enabled enantioselective C–N coupling to access chiral boron-stereogenic BODIPYs
Nature Communications (2025)