Abstract
Disentangling the limitations of O-O bond activation and OH* site-blocking effects on Pt sites is key to improving the intrinsic activity and stability of low-Pt catalysts for the oxygen reduction reaction (ORR). Herein, we integrate of PtFe alloy nanocrystals on a single-atom Fe-N-C substrate (PtFe@FeSAs-N-C) and further construct a ferromagnetic platform to investigate the regulation behavior of the spin occupancy state of the Pt d-orbital in the ORR. PtFe@FeSAs-N-C delivers a mass activity of 0.75 A mgPt−1 at 0.9 V and a peak power density of 1240 mW cm−2 in the fuel-cell, outperforming the commercial Pt/C catalyst, and a mass activity retention of 97%, with no noticeable current drop at 0.6 V for more than 220 h, is attained. Operando spectroelectrochemistry decodes the orbital interaction mechanism between the active center and reaction intermediates. The Pt dz2 orbital occupation state is regulated to t2g6eg3 by spin-charge injection, suppressing the OH* site-blocking effect and effectively inhibiting H2O2 production. This work provides valuable insights into designing high-performance and low-Pt catalysts via spintronics-level engineering.
Similar content being viewed by others
Introduction
The future of a sustainable energy supply needs innovative breakthroughs in the design of inexpensive and durable catalysts for efficient proton exchange membrane fuel cells (PEMFCs). Currently, platinum (Pt)-based catalysts are considered the most promising commercial catalysts for the cathodic oxygen reduction reaction (ORR) of PEMFCs, however, their high cost and limited reserves impede their large-scale commercialization1,2,3,4. Therefore, reducing the amount of Pt used in the catalyst without compromising the activity and durability is an urgent issue. Ordered PtM alloy nanostructures (where M is Ni5, Co6, or Fe7, among other metals8) have been considered successful catalysts for decreasing the Pt load while increasing the catalytic activity of the ORR in acidic media. Current research shows that the high performance of the PtM alloy nanostructures is regulated by the d-band center theory4, strain effect9, size effect10, and surface effect11. Despite the great progress that has been made in the development of advanced Pt-based catalysts to improve Pt utilization and mass activity towards ORR, high activity, and long life are still challenging issues for PEMFCs because some kinetic dilemmas cannot be circumvented7,12,13,14.
To achieve highly durable and active low-Pt-based electrocatalysts, several critical issues need to be addressed. (1) The Pt active sites still exhibit a tendency to strongly adsorb oxygen-containing intermediates and need higher overpotentials. (2) Their stability in acidic media is also unsatisfactory because of the H2O2 through the associative pathway; H2O2 will attack the active sites and carbon support. To address these issues, a dissociation pathway is desired to reduce the O-O bond activation energy barrier, bypassing the direct production of OOH*, and thereby avoiding the side reactions by introducing new variables (second adsorption site15,16). In this regard, developing structurally novel Pt sites to regulate the adsorption state of O2 and oxygen-relevant intermediates (OOH*, O*, and OH*) is a promising way to promote ORR kinetics in practical applications. In principle, an excellent ORR electrocatalyst should have an optimal d-orbital electronic structure of the metal sites to achieve an ideal adsorption Gibbs free energy to facilitate multistep proton-coupled electron transfer steps. Therefore, the spin and magnetic-related principle has provided a promising pathway for engineering advanced ORR electrocatalysis, because the paramagnetic oxygen species are reduced to diamagnetic intermediates via spin-electron evolution during multiple electron transfer processes17,18. Recently, tuning the spin state of single-atom Fe centres has been achieved to develop advanced Fe-N-C catalysts. Therefore, this spin occupancy state modulation strategy in which the Pt d-orbital can regulate the adsorption state of O2 and oxygen-relevant intermediates is a potential way to optimize ORR kinetics. However, to the best of our knowledge, tuning the spin occupancy state of Pt sites to accelerate the adsorption and dissociation kinetics of O2 and oxygen-relevant intermediates for developing high-performance low-Pt catalysts remains largely unexplored. Herein, to boost the performance of low-Pt catalysts, we directed the charge injection to the Pt sites with an appropriate spin configuration and optimized the adsorption behavior via magnetic changes in the interactions of the dual-Fe sites from the PtFe alloy nanocrystals and atomically dispersed FeN4 sites from a carbon substrate (denoted as PtFe@FeSAs-N-C). A combination of operando experiments and theories evidenced that the injected electrons of the Fe dz2 orbital filled the perpendicular Pt dz2 orbital and effectively achieved the side-on adsorption of O2 and the dissociative pathway (direct 4e- pathway). Moreover, the σ* bond generated between the Pt dz2 orbital and the 2p orbital of OH* accelerated the desorption of OH* and effectively inhibited the site-blocking effect, thereby facilitating the ORR kinetics. This process not only achieved electrochemical accessibility, but also restricted catalyst agglomeration and retarded the corrosion of carbon supports, metal detachment, and Ostwald ripening processes. Therefore, PtFe@FeSAs-N-C demonstrated a mass activity of 0.75 A mgPt−1 at 0.9 V and a peak power density of 1240 mW cm−2 in the full-cell assessment, outperforming commercial Pt/C catalysts (JM 20 wt%, 0.16 A mgPt−1 and 1320 mW cm−2), and a mass activity retention of 97% with no noticeable current drop at 0.6 V for over 220 h was attained; this mass activity retention exceeded the Department of Energy (DOE) technical targets for 2025.
Results
Synthesis and structural characterization
Recently, our group developed an adjacent ferromagnetic Mn (II) material that can adjust the electron spin state of the Fe (III) eg obital in N-coordinated dual-metal Mn,Fe-N6-C, which is a high-activity ORR catalysts in acidic electrolytes19. Moreover, our previous data demonstrated the potential of the PtZn alloy nanocrystalline material as a low-Pt ORR catalyst20, which, unfortunately, exhibited considerable activity loss at the rotating-disk electrode (RDE), thus, its performance under harsh fuel-cell conditions is questionable. We reasoned that the rational combination of a PtFe alloy with ferromagnetic micro-environments such as Fe-N-C materials could modulate the spin occupancy state of the Pt dz2 orbital, thereby improving the 4e- ORR kinetics. Based on the above hypothesis, we fabricated PtFe intermetallic nanocrystals on a single atomically dispersed Fe-N-C substrate (FeSAs-N-C). Briefly, as shown in Fig. 1a and S1, the FeSAs-N-C substrate was first synthesized by annealing the dual-confined Fe, Zn-based zeolitic imidazolate framework-8 (Fe/ZIF-8) at 950 °C for 2 h in an argon flow. Then, the metal salts of H2PtCl6·6H2O were dissolved in an isopropanol (reductant agent) solution mixed with FeSAs-N-C, followed by heating at 115 °C for 2 h, and the resulting powder was further annealed at 900 °C for 1 h in an argon flow to generate PtFe alloy nanocrystals on FeSAs-N-C (PtFe@FeSAs-N-C). Transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) were employed to probe the fine structure of PtFe@FeSAs-N-C. Fig. S3 clearly demonstrates that FeSAs-N-C effectively inherits the initial dodecahedral shape of ZIF-8 without any discernible metallic species. Figure 1b,c and S5 clearly show PtFe alloy nanocrystals on PtFe@FeSAs-N-C with high density and excellent size uniformity of ~2.46 nm. As shown in Fig. S4, the evident decrease in the pore size distribution of PtFe@FeSAs-N-C at ~3 nm demonstrates the successful introduction of the PtFe alloy nanocrystals through microporous confinement and alloying effects (Table S1). Meanwhile, the retained rich surface area and pore structures ensure an expedited electron/mass transfer process. This process could also enhance the binding between the Pt alloy nanocrystals and the substrate, which prevents the alloy nanocrystals from aggregating. Powder X-ray diffraction (PXRD) shows (00 l), (111), and (200) peaks in the 2θ range of 23.96°, 41.04°, and 47.02°, respectively, indicating ordered stacking along the Z direction (Fig. S2). The wide-angle diffraction peak of PtFe@FeSAs-N-C at 41.04° can be perfectly indexed to the tetragonal phase of the PtFe structure with a lattice fringe of 3.71 Å (JCPDS 43-1359).
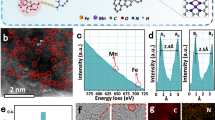
a Scheme of PtFe@FeSAs-N-C. b TEM images of the PtFe@FeSAs-N-C showing a uniform distribution of Pt nanocrystals. c HAADF-STEM image and d corresponding EELS analysis to verify the coexistence of PtFe and atomic level Fe in the PtFe@FeSAs-N-C electrocatalyst (the spots in the red dashed circles is ascribed to the Fe single atoms, inset: differences in mass content between Fe and Pt in PtFe alloy nanoparticles.). e HAADF-STEM image of an individual PtFe nanocrystals. f FFT pattern of Fig. 1e. g The enlarged HAADF-STEM image in the blue box in e. h Crystal structure of PtFe with ordered, tetragonal structure and its projection along the (001) zone axis (blue and red spheres represent Pt and Fe, respectively). i Intensity profile across the PtFe particle from (002) and (001) directions measured from h. The distance between the strong intensities matches with the separation between Pt atoms. j EDS mapping results of PtFe@FeSAs-N-C.
Figure 1c also shows that numerous isolated Fe atoms (labelled with red circles) anchored on the carbon substrate are present21. The identification of the isolated spots can be further confirmed by local electron energy loss spectroscopy (EELS) analysis (Fig. 1d)7. The HAADF-STEM images of the PtFe alloy nanocrystals (Fig. 1e and S6) indicate that the ordered intermetallic alloy nanocrystals are uniformly dispersed on FeSAs-N-C support. As shown in Fig. 1f-h, the characterized alternating bright and dim atomic columns as well as lattice distances of 0.187 nm (002) and 0.377 nm (001) reveal a standard ordered structure of the PtFe alloy, this results is, consistent with the atomic model, the HAADF-STEM image of individual alloy nanocrystals, and the XRD patterns of a tetragonal PtFe structure22. This deduction is further supported by the line intensity profile (Fig. 1i and S7). As shown in Fig. 1j, the energy-dispersive X-ray spectroscopy (EDX) mapping of PtFe@FeSAs-N-C obviously show the presence of Pt, Fe, N, and C. The microscopic characterization shows ordered alloy PtFe nanocrystals; these nanocrystals were successfully formed and anchored on the FeSAs-N-C substrate. According to the inductively coupled plasma (ICP) mass spectrometry results, the Fe and Pt loadings in PtFe@FeSAs-N-C are 7.75 and 6.97 wt%, respectively (Table S2).
Electronic states and coordination environment of PtFe@FeSAs-N-C catalyst
We studied the electron transfer behavior of Pt and Fe in the formation of PtFe@FeSAs-N-C catalysts. Fig. S8 shows the wide scan X-ray photoelectron spectroscopy (XPS) results for the samples, and Table S3 summarizes the corresponding elemental compositions, these results, confirm the presence of Pt, Fe, C, and N. The deconvolution of the Pt 4 f spectra reveals the main peaks for Pt0 along with Pt2+ (Fig. S9a). The electron transfer from Pt to the FeSAs-N-C support causes a positive shift in the Pt 4 f binding energy in PtFe@FeSAs-N-C compared to Pt/C23. The FeN4 sites embedded in the carbon substrate, which shows an electronegative behaviour,, can modify the electronic structure of adjacent carbon. The resultant electron deficiency of carbon strengthens the deposition of Pt and the metal-support interaction24. The deconvoluted N 1 s spectra of FeSAs-N-C and PtFe@FeSAs-N-C (Fig. S9b) include a dominant graphitic N along with pyridinic N, metal N, and oxidized N23,25. The existence of N-Fe peaks confirms the coordination between Fe and N in the carbon support. When Fe atoms are alloyed with Pt, the N-Fe bonds are broken, resulting in a significant decrease in the content of N-Fe and pyridinic N (Table S4). The N-Fe bonds and pyridinic N generally evidence the formation of FeN4 active sites (Fig. S10), thus, the FeN4 active site is effectively preserved for all the corresponding PtFe@FeSAs-N-C catalysts, which is consistent with the HAADF-STEM image analysis results.
X-ray absorption structure (XAS) spectra were recorded to elucidate the electronic structures of PtFe@FeSAs-N-C. No evident differences were observed in the Pt L3-edge X-ray absorption near-edge structure (XANES) spectra (Fig. 2a) in the pre-edge region compared with that of Pt foil. The stronger intensity of the white line for PtFe@FeSAs-N-C results from the electron transfer from Pt 5d to the carbon support, and further demonstrates their enhanced metal-support interaction20,26. The negative shift of the main peak from 2.51 Å (Pt foil) to 2.36 Å (PtFe@FeSAs-N-C) in the Fourier transforms of the extended XAFS (FT-EXAFS) data (Fig. S11) affirms the formation of Pt-Fe bonds. The Fe K-edge XANES spectra in Fig. 2b, the pre-edge of PtFe@FeSAs-N-C shows a shift towards lower energy compared to that of FeSAs-N-C and FePc, these results indicate the reduction of Fe3+ in the carbon matrix during the alloying process. The linear relationship between the valence of Fe and the pre-edge location shows that the valence of Fe in PtFe@FeSAs-N-C is approximately +0.92 (Fig. 2c). Moreover, the FT-EXAFS spectra of the R space show that the shoulder at 2.48 Å originates from the scattering by the Fe-Pt bond, whereas the strong Fourier transform peak at 1.53 Å is attributed to scattering from the Fe-N bond in the carbon support (Fig. 2d)27. The coordination structures of Pt and Fe are also further supported by wavelet transform EXAFS analysis (Fig. S12). Ultraviolet photoelectron spectroscopy (UPS) (Fig. 2e and S13) was performed to obtain a better understanding of the band structure. Intriguingly, the cut-off energies (Ecutoff) of PtFe@FeSAs-N-C and FeSAs-N-C are 17.59 and 16.97 eV, respectively. According to the equation of eΦ= 21.22 eV-Ecutoff17, the work functions (eΦ) of PtFe@FeSAs-N-C and FeSAs-N-C were calculated to be 3.63 and 4.25 eV, respectively; these results indicate that PtFe@FeSAs-N-C tends to donate more electrons to the O2 molecules/oxygen-relevant intermediates28. Furthermore, the energies of the highest occupied molecular orbital (HOMO) were determined to be 2.65 and 2.24 eV for PtFe@FeSAs-N-C and FeSAs-N-C catalysts, respectively, indicating that electron delocalization occurred in the Fe sites17,25. As the charge redistribution is accompanied by the transition of the 3d electron spin configuration, an electron spin resonance (ESR) test was employed to determine the spin states of the Fe in PtFe@FeSAs-N-C. As the curves displayed in Fig. 2f, the major characteristic signals at g = 4.48 are attributed to the Fe(III) medium-spin states (S = 3/2), and the minor signals at g = 2.12 and 2.09 are assigned to the Fe(III) high-spin states (S = 5/2)29,30. Meanwhile, the Fe(III) signal in the ESR spectra of PtFe@FeSAs-N-C decreases relative to FeSAs-N-C, which may be due to partial alloying of the Fe atoms. As a solid foundation, the electron-spin state of Fe in PtFe@FeSAs-N-C was measured by 57Fe Mössbauer spectroscopy (Figs. S14,15 and Table S5). Bartholomew and Boudart suggested that the Mössbauer absorption probability of the surface Fe atoms in an alloy is substantially the same as that in the bulk, especially for nanosized PtFe particles31,32. Thus, PtFe@FeSAs-N-C exhibited a dominant resonant line sextet and iron metal component, which was attributed to the formation of the PtFe alloy, and the spectral area of PtFe reached 81.5%. Besides, three quadrupole split doublets were observed at the center of the center shift: 0.84 mm s−1, 2.19 mm s−1, and 3.20 mm s−1 were ascribed to medium-spin Fe(III)N4 (11.5 %), high-spin Fe(III)N (2.1 %), and medium-spin Fe(II)N4 (4.9 %) in the FeSAs-N-C substrate, respectively. To monitor the evolution of the magnetic properties of PtFe@FeSAs-N-C as a function of Fe loading, magnetization measurements were carried out33. According to the saturation magnetizations (Fig. S16), the average magnetic moments of the individual Fe atom in FeSAs-N-C and PtFe@FeSAs-N-C were determined to be 4.96 and 4.37 μB, respectively. The decrease in the magnetic moment of the Fe atoms with low Pt loading was consistent with the ESR results; this result indicated a redistribution of the electronic structure in the catalyst, which was beneficial for the attainment of the excellent catalytic performance of PtFe@FeSAs-N-C.
Comparisons of the X-ray absorption near-edge structure spectra of PtFe@FeSAs-N-C, FeSAs-N-C, Fe foil, FePc, and Pt foil: a XANES spectra of PtFe@FeSAs-N-C and Pt foil: Pt L3 edge. b XANES spectra of PtFe@FeSAs-N-C, FeSAs-N-C, Fe foil, and FePc: Fe K edge. c Liner fitting for Fe valences in PtFe@FeSAs-N-C, FeSas-N-C, Fe2O3, and Fe foil derived from corresponding Fe K-edge XANES spectra. d FT-EXAFS spectra of PtFe@FeSAs-N-C, FeSas-N-C, Fe foil, and FePc at the Fe K-edge. e Enlarged UPS spectra. f X-band EPR spectra of PtFe@FeSAs-N-C and FeSAs-N-C.
ORR performance
The ORR electrocatalytic activity of the catalysts was evaluated by a rotating-ring disk electrode (RRDE) in an O2-saturated 0.1 M HClO4 electrolyte at room temperature, without iR correction. As shown in Fig. 3a and S17, the ORR activities were measured by linear sweep voltammetry (LSV) at a rotation speed of 1600 rpm. Obviously, PtFe@FeSAs-N-C with multiple active sites exhibits the highest ORR activity, with a half-wave potential (E1/2) of 0.872 V vs. RHE, displaying positive shifts of 102 and 43 mV relative to FeSAs-N-C and commercial Pt/C catalysts, respectively. Furthermore, the Koutecky-Levich (K-L) plots (Fig. S18) and the RRDE results (Fig. S19) confirm the 4e- transfer process for PtFe@FeSAs-N-C ( ~ 3.5%) with a lower H2O2 yield than that of FeSAs-N-C ( ~10%). As shown in Fig. S20, PtFe@FeSAs-N-C exhibits a kinetic current density of 25.82 mA cm−2 at 0.85 V vs. RHE, this density is greatly improved with respect to FeSAs-N-C (2.15 mA cm−2) and Pt/C (11 mA cm−2). The mass activity and specific activity (SA) of PtFe@FeSAs-N-C are 1.18 A mgPt−1 and 3.64 mA cm−2, respectively, at 0.85 V vs. RHE, these are superior to those of commercial Pt/C (0.21 A mgPt−1, 334 mA cm−2) catalysts (Figs. S21 and S22). As shown in Fig. S23, PtFe@FeSAs-N-C (11.2 Ω) shows lower resistance than both FeSAs-N-C (17.1 Ω) and Pt/C (18.2 Ω), indicating the effective promotion of rapid electron/proton transfer. Specifically, to understand the origin of the excellent ORR performance of PtFe@FeSAs-N-C, anionic SCN- (10 mM) poisoning measurements were carried out in 0.1 M HClO4 electrolyte (Fig. 3c). SCN- has a strong affinity for Fe ions and is used as a probe to poison the Fe-N4 sites34. As a result, the slight decrease of E1/2 reveals that the single-atom FeN4 site around the alloy nanocrystals also exhibits certain activity; however, E1/2 is still greater than 0.81 V vs. RHE, indicating that the PtFe alloy nanocrystals are responsible for the high ORR activity of PtFe@FeSAs-N-C. The Fe atoms in the support mainly provide Fe sources for alloying and anchoring PtFe nanocrystals to inhibit agglomeration and demetallization.
a Linear sweep voltammetry polarization curves under a rotation rate of 1600 rpm for PtFe@FeSAs-N-C after 30 k and 50 k potential cycles (0.6-1.0 V vs. RHE) in O2-saturated 0.1 M HClO4 electrolyte at 25 °C (without iR compensation, catalyst loading: 0.1 mg cm−2). b The mass activity loss ratio of PtFe@FeSAs-N-C and Pt/C at 0.85 V after 30 k and 50 k potential cycles. c Linear sweep voltammetry polarization curves under a rotation rate of 1,600 rpm for PtFe@FeSAs-N-C in O2-saturated 0.1 M HClO4 electrolyte at 25 °C before and after the addition of 10 mM SCN- (without iR compensation). d UV-Vis absorption spectra of 0.1 M HClO4 electrolyte of ABTS + H2O2 with PtFe@FeSAs-N-C, FeSAs-N-C catalyst and no catalyst. Inset: the absorbance value of the solution at 417 nm. e, f The comparison of in-situ FTIR spectra under applied potentials among PtFe@FeSAs-N-C, Pt/C, and FeSAs-N-C. g Proposed ORR reaction pathways occurring on the Pt site in PtFe@FeSAs-N-C (blue and red spheres represent Pt and Fe, respectively).
Next, we assessed the long-term stability of PtFe@FeSAs-N-C in an O2-saturated 0.1 M HClO4 electrolyte. The E1/2 of PtFe@FeSAs-N-C (Fig. 3a) decreases by only 9 mV after 50,000 potential cycles between 0.6 and 1.0 V vs. RHE potential windows, this is, much lower than those of FeSAs-N-C (71 mV) and Pt/C (88 mV) (Fig. S24). Accordingly, the retained mass activity of PtFe@FeSAs-N-C is 1.031 A mgPt−1 and 0.928 A mgPt−1 after 30,000 and 50,000 potential cycles, respectively; specifically, the losses are 12.5% and 9.9% respectively, in the mass activity, and these losses are much lower than the 70.7% and 48.5%, respectively, of Pt/C (Fig. 3b). To evaluate the stability of the catalysts at a more practical temperature, the temperature of the testing system was increased to 60 °C. Notably, after 30,000 potential cycles, PtFe@FeSAs-N-C still demonstrates high stability, with a minimal E1/2 loss of only 28 mV (Fig. S25) and 70% mass activity retention, while the Pt/C catalyst suffers more severe degradation of ORR activity (Fig. S26), with only 17% mass activity retention (inset in Fig. S25a). All the above results verify that PtFe@FeSAs-N-C has greater activity and greater stability than FeSAs-N-C, Pt/C, and previous reports of Pt-based electrocatalysts (Fig. S25b).
One of the major concerns with M-N-C catalysts in fuel cells is the formation of a large amount of reactive oxygen species (ROS, e.g. H2O2) as a byproduct, which is detrimental to the membrane and ionomers35,36. As reported, 2,20-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) can be oxidized by ROS, resulting in a change in absorbance at ~417 nm in UV-Vis absorption spectroscopy, this, is mainly manifested as the solution changes from colourless to green (Fig. 3d and S27)37. As expected, the UV-Vis absorbance intensity of FeSAs-N-C + H2O2 + ABTS is approximately 3 times that of PtFe@FeSAs-N-C + H2O2 + ABTS, thus, the introduction of Pt can significantly inhibit the Fenton reaction and the accumulation of ROS. This deduction is consistent with previous studies, showing mixing Pt38 and/or Pt-Co alloy6 particles as peroxide/radical scavengers with M-N-C can alleviate H2O2 accumulation.
The morphology and structure of PtFe@FeSAs-N-C after the stability tests in the RRDE were further analyzed by HAADF-STEM and STEM-EELS. As shown in S28, after 50,000 potential cycles, the sizes of the PtFe nanocrystals are effectively preserved at 3.35 nm without apparent agglomeration. These results support our hypothesis that the existence of the FeN4 sites in the supports strengthens the metal-support interaction, leading to less detachment and agglomeration of the PtFe nanocrystals. Also, abundant single atoms are still uniformly distributed in the carbon support (Fig. S29a). The lattice spacing and ordered structure characterized in Fig. S29b are consistent with those of the PtFe nanocrystals in the catalyst before potential cycling (Fig. 1e), because of the ordered tetragonal crystal structure. The EELS analysis (Fig. S29c) verified the preservation of single Fe atoms with an N-coordinated configuration, similar to that of the pristine sample (Fig. 1d). Significantly, the PtFe nanocrystals on FeSAs-N-C support maintain their intermetallic compound structures due to the strong metal-support interaction (Fig. S29d), which plays an important role in maintaining the stability of PtFe@FeSAs-N-C. To better demonstrate that the strong metal-support interaction and the formation of ordered PtFe alloys can effectively suppress the leaching of Pt and Fe metals, XPS characterization was used to measure the retention rates of metal Pt and Fe metals in the catalyst after stability testing. As shown in Fig. S30, after 30,000 potential cycles, for FeSAs-N-C, the amount of leached Fe is as high as 45.1%, while it is greatly reduced to 14.2% for PtFe@FeSAs-N-C. For Pt/C, the amount of leached Pt is as high as 63%, whereas it is only 12.5% for PtFe@FeSAs-N-C. Furthermore, the excellent stability of the PtFe structure was confirmed by comparing the ICP-MS, Raman, and XRD results of the catalyst before and after potential cycling (Figs. S31,32 and Table S6).
Catalytic mechanism analysis
In-situ FTIR, operando XAFS, and operando magnetometry techniques were employed to elucidate the catalytic mechanism of the multistep ORR based on spintronic behavior and bond order theory. As shown in Fig. 3e,f and S33, Pt/C and FeSAs-N-C exhibit a readily identifiable OOH* peaks at 1226 and 1234 cm−1, and strong O-O stretching modes of O2,ad at 1462 and 1469 cm−1, respectively, with increasing intensity caused by decreasing the applied potentials; this shows an associative pathway of Pt/C and FeSAs-N-C39. However, the OOH* peak of PtFe@FeSAs-N-C (1237 cm−1) is significantly redshifted and attenuated compared with that of Pt/C, indicating that the 4e- reaction pathway changed from the indirect pathway (association pathway) of the Pt/C catalyst to the direct pathway (dissociative pathway, Griffiths adsorption model). As shown in Fig. 3g, this path does not generate OOH * and can strongly inhibit the production of byproducts, which is beneficial for improving the ORR stability.
The real-time magnetic responses accompanying the electrochemically-driven reactions were recorded to investigate the magnetization evolution during the ORR process. As shown in Fig. 4a and S34, the magnetization and effective magnetic moment of PtFe@FeSAs-N-C gradually decrease as the applied potential decreases from 1.1 to 0.2 V vs. RHE; this decrease is attributed to the Griffiths adsorption model of O2 molecules and the optimization of O2 molecule/intermediate adsorption behavior on the active Pt site40. Kelvin probe force microscopy (KPFM) was used to probe the variations in eΦ between PtFe nanocrystals and FeSAs-N-C41. KPFM was conducted with a derivative imaging mode of atomic force microscopy to monitor the changes in the local eΦ. The calibration standard sample was highly oriented pyrolytic graphite (HOPG, eΦ = 4.6 eV). As shown in Fig. S35, the topographical image and contact potential difference (CPD) image of PtFe@FeSAs-N-C were simultaneously recorded, and the corresponding acquired profiles were also obtained. The CPD of the PtFe nanocrystals is approximately −100 mV (eΦ = 4.8 eV) lower than that of the probe, while that of FeSAs-N-C is 300 mV (eΦ = 4.4 eV) greater than that of the probe, implying that electrons tend to flow from the FeSAs-N-C support to the PtFe nanocrystals. Moreover, due to the electronegativity difference between Pt (χ = 2.28) and Fe (χ = 1.83) metal atoms, electrons have a driving force to transfer from Fe to Pt. Specifically, this is further confirmed by operando XANES of the Pt L3-edge and Fe K-edge. In operando XANES of the Pt L3-edge (Fig. 4b), with the ORR process, and given the applied potential decreasing from 1.1 to 0.0 V, the white line intensity continuously decreases, revealing an increase in the electron occupied state of the Pt 5d orbital. As expected, operando XANES of the Fe K-edge shows the opposite phenomenon (Fig. S36). When the applied potential decreases from 1.1 to 0.0 V vs. RHE, the absorption edge continuously shifted towards high energies; thus, Fe in PtFe@FeSAs-N-C lost electrons and the valence state significantly increased during the ORR process. The valence state fitting of ex-situ and operando Pt L3-edge XANES also confirmed this result (Fig. S37). To our best knowledge, the strong bonding of OH* over the Pt sites leads to sluggish ORR kinetics, which is a common challenge for Pt-based catalysts16. Therefore, the orbital interactions between the Pt sites and OH* were then investigated. As reported, in 0.1 M HClO4 media, the ORR kinetically slow O* → OH* reduction peak is located between 0.55 and 1.0 V vs. RHE42. As shown in Fig. 4a, b, S36, and 37, when the potential decreases from 1.1 to 0.5 V vs. RHE, the intensity of the white line representing the Pt L3-edge decreases, the absorption edge of the Fe K-edge positively shifts and the effective magnetic moment significantly decreases, which strongly confirms prove that the number of Pt 5d electrons occupied increases when they interact with OH*.
a The M-T curve and effective magnetic (μeff) moment of the PtFe@FeSAs-N-C vary with the applied potential in the O2-saturated 0.1 M HClO4 electrolyte. b Operando Pt L3-edge XANES spectra for PtFe@FeSAs-N-C in 0.1 M HClO4. c The orbital interactions between Pt site and the ORR (O* and OH*) intermediates on PtFe@FeSAs-N-C and Pt single site. d A schematic diagram of Pt-Fe spin charge injection effect for O2 activation.
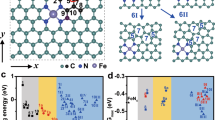
The bond order theorem was applied to describe the potential orbital interactions between the adsorption intermediates and Pt sites at the electrocatalyst surface. According to the principle of symmetry conservation, we omitted the orbitals (dx2-y2 and dxy) of Pt as shown in Fig. 4c, d. Since the orbital interaction strength between Pt and the oxygen-containing intermediates is proportional to the bond order, we calculated the bond order of the O* and OH* intermediates bound to Pt in PtFe@FeSAs-N-C using the bond order = (A-B)/2 formula, where A and B are bonding electrons and antibonding electrons, respectively. Underorbital interactions, the bond order between Pt sites and adsorbed state O* is 1.5, which is 1 greater than the bond order between Pt and OH*, indicating that the adsorption of O* by Pt sites is significantly stronger than that of OH*. From a thermodynamic perspective, this is conducive to the desorption of the products. Notably, this phenomenon is opposite at the Pt single site16. This occurs because the production of an additional σ* bond between the fully occupied Pt dz2 orbital of PtFe@FeSAs-N-C and the π orbital of the OH* intermediates, results in a weakened Pt-OH* bonding strength; this is beneficial for suppressing the site-blocking effect, and effectively facilitating the ORR kinetics. Moreover, O2 temperature-programmed desorption (O2-TPD, Fig. S38) measurements were also performed to determine the influence of the PtFe nanocrystals on the catalytic performance. The Pt-O peak of PtFe@FeSAs-N-C shows a desorption temperature comparable to that of Fe-O of FeSAs-N-C, but with a stronger intensity of the O2 desorption peak, which implies that the Pt site in PtFe@FeSAs-N-C has a strong adsorption affinity for O2, which is favourable for the activation of the O-O bond. Consequently, the above results powerfully confirm that the spin charge injection effect between Pt and Fe can achieve the Griffiths model ORR pathway on PtFe@FeSAs-N-C, preventing the site-blocking effect and formation of H2O2 to enhance both selectivity and stability.
Density functional theory (DFT) calculations were performed to further explore the origin of the high activity and durability of PtFe@FeSAs-N-C. Based on the experimental results, the optimization of the catalytic reaction behavior at the Pt site is derived from the bidirectional regulation of Fe atoms in the substrate and alloy; thus, PtFe(111), Pt(111)/FeN4, Pt(111), and FeN4, simulation models were constructed to represent the possible types of active sites (Fig. S39 and supplementary data 1). Periodic stacked magnetic multilayer structures consisting of two magnetic layers separated by a nonmagnetic spacer (i.e., Pt), are known to exhibit special interlayer exchange coupling interactions43. In this study, the hybridized d-orbitals of Pt and Fe constitute the bypass transfer of magnetization between the different layers throughout the entire structure44. This Pt-Fe hybrid involving 3d-5d orbitals can be clearly seen in the density of states (DOS) of PtFe(111) structure (Fig. S40). Furthermore, the spin density distribution of the PtFe(111) structures was calculated to confirm the polarization of Pt atoms by adjacent Fe atoms, with an average magnetic moment of 0.371µB (Fig. S41). To verify the intrinsic driving mechanism of the electron structure of the Pt site regulated by Fe atoms in the substrate, we calculated the eΦ of the PtFe(111) and FeN4 structural models. The energy level of eΦ determines the direction and possibility of electron transfer, and the electrons can more easily leave the metal when the eΦ value is lower.. As shown in Fig. 5a and S42, the eΦ of FeN4 (4.02 eV) is significantly lower than that of PtFe(111) (4.93 eV) and consistent with KPFM measurements. Therefore, in PtFe@FeSAs-N-C, a built-in electric field is formed at the interface of the PtFe cluster and FeSAs-N-C, and electrons act as a driving force from FeSAs-N-C with low eΦ (4.02 eV) to the PtFe cluster with high eΦ (4.93 eV)41, thus, this process jointly regulates the adsorption and desorption behavior of Pt sites towards O2 and oxygen-relevant intermediates with Fe atoms on the surface of clusters.
a The work function (eΦ) of PtFe (111). b Gibbs free energy diagram of ORR on PtFe (111), Pt (111)/ FeN4, Pt (111), and FeN4 at U = 0 V. c Gibbs free energy diagram of ORR on PtFe (111), Pt (111), and FeN4 at U = 0.85 V. d Energy barrier for OH* protonation to form H2O. e Gibbs free energy diagram of ORR on Pt (111)/ FeN4, Pt (111), and FeN4 at U = 0.85 V. (dark blue, rose red, blue, red, gray, and white spheres represent Pt, Fe, N, O, C, and H atoms, respectively).
To evaluate whether the ORR dissociative pathway occurs in various simulation models, the key intermediates of O + OH*, 2OH*, and OH* (*denotes the adsorbed state) were optimized. The determined potential energy profiles show that PtFe(111) (0.54 eV) and Pt(111)/FeN4 (0.56 eV) possess a lower overpotential than Pt(111) (1.11 eV) and FeN4 (0.74 eV), which confirmed the better catalytic performance of PtFe@FeSAs-N-C (Fig. 5b). Notably, the formation and dissociation of O + OH* intermediates efficiently result in more favourable direct 4e- processes (Fig. S43). However, these two processes are not easily performed on the FeN4 and Pt(111) surfaces because O + OH* cannot spontaneously form according to thermodynamics; its formation, would greatly affect the catalytic reaction process and selectivity. Corresponding to the potential of activity evaluation in the experiment, the Gibbs free energy diagrams of the ORR on two possible active sites were constructed at 0.85 V. As shown in Fig. 5c-e, PtFe(111) and Pt(111)/FeN4 exhibit excellent ORR activity, both with rate-determining steps (RDS) at OH* protonation, and reaction energy barriers of only 0.16 and 0.18 eV; these barriers are much smaller than those for Pt(111) and FeN4 (RDS are the second OH* protonation, with energy barriers of 0.73 and 0.32 eV, respectively), and they alleviate the site-blocking effect of OH*, to facilitating the ORR kinetics. Based on the above results, we constructed a PtFe(111)/FeN4 structural model to theoretically verify the experimental results. First, the adsorption energy of PtFe(111) on the FeN4 support was calculated, and the results showed that the adsorption energy was −0.792 eV, which confirmed the stability of the PtFe(111)/FeN4 structure. Second, the charge density difference of the PtFe(111)/FeN4 structure was calculated, as shown in Fig. S44. Clearly, charge aggregation occurs between PtFe(111) and the FeN4 support, which suggests a strong metal-support interaction between the PtFe(111) nanoparticles and the FeN4 support. The step diagram of the 4e- ORR carried out on the surface of the PtFe(111)/FeN4 model was further calculated, as shown in Fig. S45. For the PtFe(111)/FeN4 structure, the RDS is also the first OH*protonation, as experimentally confirmed, its RDS energy barrier is the lowest of all structures at only 0.033 eV, confirming that the PtFe(111)/FeN4 structure is more favourable for the 4e- ORR.
Therefore, the strong metal-support interactions and spin-charge injection effects between Pt and Fe atoms are beneficial for ORR enhancing activity and stability by optimizing O2 molecule activation/dissociation and the OH* desorption process. For single-atom FeN4 sites, the inappropriate adsorption of oxygen-related intermediates (O2*, OH*, and OOH*) on the active site can easily lead to active site poisoning and corrosion problems, resulting in sluggish kinetics(Fig. S46)13,45. Compared to Pt(111) and FeN4 sites, the PtFe(111)/FeN4 structure exhibits a unique ORR catalytic mechanism, with the optimal adsorption state of oxygen intermediates and the ability to inhibit the occurrence of side reactions. Combined with the low RDS, the OH* protonation energy barrier of PtFe(111)/FeN4 can effectively improve the ORR kinetics of PtFe@FeSAs-N-C and increase its stability.
Applications in the PEMFC system
PtFe@FeSAs-N-C, FeSAs-N-C, and commercial Pt/C catalysts were equipped into the membrane electrode assembly (MEA) as a cathode to evaluate fuel cell performances under 28 psi H2-O2 environment (Fig. 6a, b and S47). Compared with FeSAs-N-C (0.54 W cm−2) and typical Pt/C (1.38 W cm−2, 0.2 mgPt cm−2), the fuel cell with PtFe@FeSAs-N-C shows significantly enhanced performance with a power density of 1.24 W cm−2 (0.12 mgPt cm−2). The PtFe@FeSAs-N-C cell in a H2-air environment (Fig. 6c) shows a better performance than that of FeSAs-N-C in the whole current density range with a higher peak power density (0.71 vs. 0.42 W cm−2), this, also significantly outperformed the 2021 DOE milestone, and achieved current densities of 240 mA cm−2 at 0.8 V ( >60 mA cm−2) and 690 mA cm−2 at 0.675 V ( ≥300 mA cm−2)46. The mass activity of PtFe@FeSAs-N-C calibrated to an absolute H2 and O2-air pressure of 28 psi from H2/O2 and H2/air polarization curves is 0.75 and 0.70 A mgPt−1 at 0.9 ViR-free, respectively; these values are around 4.6 times greater than that of Pt/C (0.16 A mgPt–1), and 1.7 times higher of the 2025 DOE activity target (0.44 A mgPt−1) (Fig. 6e)26. The mass activity of PtFe@FeSAs-N-C is higher than those of most Pt-based and hybrid electrocatalysts (Table S8). In addition, PtFe@FeSAs-N-C cell was subjected to an accelerated durability test according to the DOE protocols7. As shown in Fig. 6d and S48, the i-v polarization curve shows no significant decay in current density after 30,000 continuous cycles, with low voltage decreases of only 6 mV (H2-O2) and 2 mV (H2-air) at a current density of 0.8 A cm−2; these results show the excellent durability of PtFe@FeSAs-N-C. The mass activity at 0.9 ViR-free slightly decreases to 0.73 A mgPt−1 even after 30,000 cycles in H2-O2 environment; this corresponds to only a 3% decrease. Additionally, this result surpasses the 2025 DOE durability goal of less than 40% mass activity loss after 30,000 cycles26. A chronoamperometric test under H2-O2 at 0.6 V was also conducted for both the anode and cathode to further evaluate the long-term durability of the different cathode catalysts (Fig. 6f). The fuel cell with a PtFe@FeSAs-N-C cathode shows a nearly constant current density over 220 h in the H2-O2 environment. In contrast, the current density of the Pt/C fuel cell drops by 60% in 120 h. Both the square-wave potential-cycling and constant-voltage tests confirm that PtFe@FeSAs-N-C has better fuel cell durability than the Pt/C, Pt-based, and other hybrid electrocatalysts (Fig. 6g and Table S8)6,7,23,26,46,47,48,49,50,51. The TEM images after the H2-O2 stability test show (Fig. S50) that the original morphology and elemental distribution remain well preserved, and no obvious particle agglomeration occurs after more than 220 h of operation, which further confirms the structural stability of PtFe@FeSAs-N-C catalyst.
a The structural components of a single PEMFC. b H2/O2 and c H2-air fuel cell polarization (left axis) and power density (right axis) plots with loadings of 0.2 mgPt cm−2 for Pt/C, 0.12 mgPt cm−2 for PtFe@FeSAs-N-C, and 4 mg cm−2 catalyst loading for FeSAs-N-C in the cathode under H2/O2 flow at 80 °C, 28 psi. d H2/O2 fuel cell polarization (left axis) and power density (right axis) plots of the PtFe@FeSAs-N-C cathode before (BOT) and after 30,000 potential cycles between 0.6 and 0.95 V. e Mass activity (MA), MA test under H2/O2 flow at 80 °C, 28 psi. f Current density retention as a function of time for PtFe@FeSAs-N-C (0.12 mgPt cm−2) and Pt/C (0.2 mgPt cm−2) at a constant potential of 0.6 V under H2/O2 flow at 80 °C, 0 psi. g Comparison of MA retention after square wave accelerated durability testing between PtFe@FeSAs-N-C with the state-of-art in the literature.
Discussion
In summary, we constructed a stable low-Pt-based electrocatalyst consisting of ultrafine PtFe nanocrystals on FeSAs-N-C with a well-optimized spin occupancy state for the ORR. With the proposed directional spin charge injection effect, the Pt dz2 orbital occupation state of PtFe@FeSAs-N-C is well-regulated by the dual-Fe sites from the ordered PtFe alloys and an atomically dispersed Fe-N-C support. As a result, PtFe@FeSAs-N-C has a high mass activity (0.75 A mgPt−1) at 0.9 ViR-free, high peak power density of 1.24 W cm−2, and an excellent rated power of 10.3 W mgPt−1 during PEMFC tests. More importantly, PtFe@FeSAs-N-C demonstrates an extraordinary durability, with a high mass activity retention of 97%, a voltage loss of only 6 mV at 0.8 A cm−2 after 30,000 cycles, and no noticeable current decrease at 0.6 V over 220 h. We developed a combination method using a real-time magnetic response recorder-electrochemically-reaction, operando spectroscopy, and theories to confirm the 4e- dissociative reaction pathway through Griffiths adsorption; this method, addressed the limitations of difficulty of O-O bond activation and OH* site-blocking effects on the Pt sites, which accelerated the reaction kinetics and decreased the ORR overpotential to an ideal value. Moreover, bypassing H2O2 greatly improved the durability of PtFe@FeSAs-N-C. Our study provides many opportunities for designing low Pt catalysts, in which the selective evolution of different reactive oxygen species can be rationally tuned for highly efficient practical PEMFCs.
Methods
Chemicals and reagents
All chemicals were purchased and used without further purification. Iron(III) acetylacetonate (Fe(acac)3, 98%, Innochem), zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 99%, Alfa Aesar), 2-methylimidazole (99%, Aladdin), methanol (AR), ethanol (AR) and isopropanol ((CH2OH)2, AR) were purchased from Sinopharm Chem. Reagent Co., Ltd. Nafion D-520 dispersion (5% w/w in water and 1-propanol, Alfa Aesar), commercial Pt/C (20 wt % Pt, Johnson Matthey), The ultrapure water (18.2 MΩ·cm) used in all experiments was obtained through ionexchange and filtration.
Synthesis of Fe/ZIF-8 and ZIF-8
In the typical synthesis, 952 mg Zn(NO3)2·6H2O and 88 mg Fe(acac)3 were dissolved in 12 mL of methanol with stirring for 20 min, and then 24 mL methanol containing 1.05 g 2-Methylimidazole was added under vigorous stirring for another 10 min at room temperature. Subsequently, the mixture was transferred into a 50 mL Teflon-lined stainless-steel autoclave for reaction at 120 °C for 4 h. After cooling to room temperature, the product was collected by centrifugation and washed with methanol four times, and finally dried at 60 °C in a vacuum oven. Individual ZIF-8 was synthesized by the same method without Fe(acac)3.
Synthesis of FeSAs-N-C and NC
The dried Fe/ZIF-8 powder was added into a ceramic boat and heated in a quartz tube at 950 °C for 2 h under an Ar atmosphere with a heating rate of 5 °C min−1. The sample NC was prepared in the same procedure by adding ZIF-8.
Synthesis of PtFe@FeSAs-N-C
The PtFe nano alloys were deposited on FeSAs-N-C25,52. At first, 16 mg FeSAs-N-C was dispersed into 8 mL of (CH2OH)2 solution containing 20 mg NaOH under continuous ultrasound. Then, 8 mL of (CH2OH)2 solution containing 10 mg H2PtCl6·6H2O was added into the above uniformly dispersed black solution under continuous ultrasound, and then vigorously stirred with magnetic force for 30 min. The obtained mixed solution was heat treated in an oil bath at 115 °C for 2 h under continuous stirring. After natural cooling, the PtFe@FeSAs-N-C precursor were collected by filtration washed of the resulting solution with ultrapure water, and finally freeze-dried. In order to convert the PtFe alloy precursor into structurally ordered PtFe intermetallic nanocrystals, the PtFe@FeSAs-N-C precursor was further annealed at 900 °C for 1 h under a flowing argon atmosphere at a heating rate of 10 °C min−1, and then naturally cooled to room temperature to obtain PtFe@FeSAs-N-C.
Physical characterizations
The structure of the samples was characterized by a transmission electron microscope (TEM, FEI Tecnai G220) and a field-emission scanning electron microscope (FE-SEM, JEORJSM-6700F). The HAADF-STEM images were obtained using a JEOL JEM-ARM200F at an accelerating voltage of 200 kV. Powder X-ray diffraction (PXRD) patterns were collected using a Y-2000X-ray diffractometer using copper Kα radiation (λ = 1.5406 Å) at 40 kV and 40 mA52. The X-ray photoelectron spectroscopy (XPS) measurements were performed with an ESCA LAB 250 spectrometer using a focused monochromatic Al Kα line (1486.6 eV) X-ray beam with a diameter of 200 μm. The Raman measurements were taken at 532 nm on a Renishaw Microscope System 16 RM2000. UPS measurements were also carried out on an ESCA LAB 250 Xi spectrometer with He I resonance lines (21.22 eV). The Fe K-edge and Pt L3-edge XANES and the EXAFS were investigated at the BL14W beamline of Conducted at Shanghai Synchrotron Radiation Facility (SSRF) (Shanghai, China). In the fluorescence mode using a fixed-exit Si (111) double crystal monochromator. The Pt and Fe content was conducted on an inductively coupled plasma mass spectrometry (ICP-MS, Thermo Scientific iCAP™ RQ). The O2-temperature-programmed desorption (TPD) of the samples was measured using Micromeritics AutoChem 2950 HP. The catalyst was first pretreated at 150 °C, purged with He for 2 h, cooled to room temperature. Then, purge with 5% O2/He at 25 °C for 2 h. Finally, the desorption curve of O2 was recorded online in He atmosphere.
In-situ FTIR measurements
In-situ FTIR measurements refer to previous reports53. Detailed, In-situ surface-enhanced Fourier transform infrared absorption spectroscopy (FTIR) was employed on a Thermo Scientific Nicolet iS50 with the MCT detector. The original diagram of the device is shown in Fig. S51. In this test, the catalyst was loaded onto the surface of a silicon column with a gold-plated thin layer as the working electrode. In-situ electrochemical testing was conducted in 0.1 M HClO4 saturated with O2, using Pt net and Ag/AgCl electrode as the counter electrode and reference electrode, respectively. The test ranges from 0 V to 1.05 V, and the operation time for each potential is approximately 5 minutes. The spectra collected in OCP (without additional bias) are used as baselines. All spectra are analyzed after subtracting the baseline. According to the Nernst equation (ERHE = EAg/AgCl + 0.0592 pH + 0.197 V), calibrate all potentials relative to RHE.
Operando XAFS measurements
All XAFS data were collected at 1W1B station of Beijing Synchrotron Radiation Facility, China15. In this operando test, a customized three electrode system was used, and the original equipment diagram is shown in Fig. S52. The working electrode is composed of 1 × 1 cm2 carbon paper loaded with catalyst, the counter electrode and reference electrode are carbon rod and Ag/AgCl electrode, respectively, and O2 saturated 0.1 M HClO4 solution is used as the electrolyte. The test potential ranges from 0 V to 1.1 V, covering the entire oxygen reduction reaction potential window. After running for 3 minutes at each potential, data spectral lines are collected. According to the Nernst equation (ERHE = EAg/AgCl + 0.0592 pH + 0.197 V), calibrate all potentials relative to RHE.
Operando magnetometry
The temperature magnetic susceptibility curves measurements, expose the sample to a temperature range of 5 to 300 K at a rate of 4 K/min, while applying an external field of 5 kOe17. The electrochemical measurements were carried out in an argon-filled glove box. Using a three-electrode system with a 1 × 0.3 cm2 carbon paper loaded with PtFe@FeSAs-N-C catalyst as the working electrode, the counter electrode and reference electrode are Pt film and Ag/AgCl, respectively, and O2-saturated 0.1 M HClO4 solution as the electrolyte. According to the Nernst equation (ERHE = EAg/AgCl + 0.0592 pH + 0.197 V), calibrate all potentials relative to RHE. The working electrodes obtained from each constant potential test are quickly transferred to the comprehensive physical property measurement system (PPMS, Quantum Design) under inert gas protection for magnetic performance testing. The magnetization values given in emu g−1 are defined per unit weight of materials. The linear magnetic background signals from the carbon paper is deducted from the total magnetic moment.
Electrocatalytic measurement
The electrochemical performance evaluation was conducted on the CH Instruments 760E electrochemical workstation54. All the electrochemical measurements were conducted in a three-electrode system, the counter electrode and reference electrode are Pt film and Ag/AgCl, respectively. According to the Nernst equation (ERHE = EAg/AgCl + 0.0592 pH + 0.197 V), calibrate all potentials relative to RHE. The working electrode was obtained by mixing 2 mg of the catalyst in 1.0 mL of a solution containing 670 μL isopropanol, 290 μL ultra-pure water, and 40 μL 5% Nafion solution, followed by ultrasonic treatment for 30 min to form uniform inks that were drop-cast onto the GC-RDE and air-dried. The area of the GC working electrode is 0.19625 cm2. 10 μL of catalyst ink was then pipetted on the GC surface, leading to a loading of 0.1 mg cm−2. Pt/C (20 wt%) electrode was prepared by using the same procedure. 0.1 M HClO4 solutions saturated with O2 were employed as the electrolyte for ORR. The electrolyte is prepared and used immediately. Take a quantitative amount of concentrated perchloric acid (HClO4, 70%, 99.999% trace metals basis, Sigma-Aldrich) and add it to a volumetric flask to dilute to 0.1 M before use.
All electrochemical measurements were conducted at room temperature (25 °C) using a rotating disk and rotating ring-disk electrode (RDE and RRDE, Pine Instrument)55. All data ensures repeatability and is presented in this work only after multiple measurements to ensure consistency. Linear sweep voltammetry (LSV) curves were obtained to investigate the performance of the catalysts at a scan rate of 5 mV s−1 with a rotating speed of 1600 rpm, the LSV curves were recorded without iR compensation, and all currents were corrected by deducting the background current that measured in N2-saturated electrolyte. Electrochemical impedance spectroscopy (EIS) is measured at open circuit voltage with a low frequency of 0.1 Hz, a high frequency of 1000000 Hz, and an amplitude of 0.005 V. The resistance values of the catalysts are the average of the electron transfer resistance obtained through multiple EIS tests.
The accelerated durability test was conducted in O2-saturated 0.1 M HClO4 solution at a scan rate of 50 mV s−1 with a potential between 0.6 V and 1.0 V vs. RHE for 30,000 and 50,000 cycles.
The electron transfer number (n) and kinetic current density (JK) were calculated from the Koutecky-Levich (K-L) equation56:
Where J is the measured current density. B is determined from the slope of the K-L plot. JL and JK are the diffusion-limiting current densities and kinetic current densities, n is the transferred electron number, F is the Faraday constant (F = 96485 C mol−1), C0 is the O2 concentration in the electrolyte (C0 = 1.26 × 10−6 mol cm−3), D0 is the diffusion coefficient of O2 (D0 = 1.93 × 10−5 cm2 s−1), and v is the kinetic viscosity (v = 0.01009 cm2 s−1).
The HO2-% and transfer number (n) were calculated by the following equations57:
where ID and IR are disk current and ring current, respectively. N = 0.4 represents the ring collection efficiency.
Calculation of unpaired d electrons for Fe ions in catalysts
According to the μeff = \(\sqrt{n(n+2)}\)μB equation, evaluate the unpaired electron number (n) of Fe in the PtFe@FeSAs-N-C and FeSAs-N-C from the effective magnetic moment (μeff). The μeff was evaluated based on the Langevin theory, μeff = \(\sqrt{8C}\)μB, where C is the Curie constant and the slope of the curve of χ−1-T according to the Curie-Weiss law χ = C/(T-Θ), χ and Θ are the susceptibility and Curie-Weiss temperature, respectively. The χ can be obtained derived from the χ = M/H equation, where M and H are the magnetization and magnetic field intensity, respectively.
Fenton-like reactivity measurements
The production of intermediate hydrogen peroxide was evaluated via a reported method37. Briefly, the metal ions were ultrasonically dissolved in 0.1 M HClO4 solution respectively to achieve a 40 μg mL−1 homogeneous suspension. The desired amounts of 2,20-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS), and hydrogen peroxide were sequentially added to reach the concentrations of 2 mM and 20 mM, respectively. After 5 min, the solution was diluted with 0.1 M HClO4 (3:100) and characterized by UV-Vis spectroscopy (SHIMADZU UV-1900 Series UV-Vis Spectrophotometer).
Fabrication and tests of H2-O2 fuel cells
The cathode catalyst ink includes a catalyst, Nafion (5%), isopropanol, and ultrapure water58. After mixing, it is sonicated in an ice water bath for 2 h to form a uniform mixture, which is then sprayed onto a gas diffusion layer (GDL).The Pt@FeSAs-N-C cathode with 0.12 mg cm−2 Pt loading. The FeSAs-N-C cathode with a 4 mg cm−2 catalyst loading and a commercial Pt/C cathode with a 0.2 mg cm−2 Pt loading were prepared using the same protocol. Commercial Pt/C-coated GDL with a 0.2 mg cm−2 Pt loading was used as the anode. Use the commercial fuel cell testing system (SMART2) to evaluate fuel cell performance in a single cell. The MEA was sandwiched between two graphite plates with single serpentine flow channels. The cell was operated at 80 °C with a back pressure of 28 psi. Pure H2 and O2/air, with 100% relative humidity (RH), were supplied to the anode and cathode at a gas flow rate of 300-800 ml min−1. The polarization curves were recorded at H2 and O2/air flow rates of 300 and 400 ml min−1, respectively, and 28 psi back pressures. Square-wave potential cycles are performed at 0.6 to 0.95 V with a sweep speed of 50 mV s−1, following the DOE protocol for evaluating the durability of catalysts7. Durability was assessed by chronoamperometric measurements at a constant discharge voltage of 0.6 V, H2/O2 flow rate of 300/400 ml min-1, 0 psi back pressure. Rated power measured at 0.67 V49,59.
DFT calculations
The spin-polarized density functional theory (DFT) calculations were conducted using the Vienna ab initio simulation package (VASP)60 with the projector augmented wave (PAW)61 pseudopotentials. The Hubbard-U correction (DFT + U method) was applied to improve the description of localized metal d-electrons in the single atom Fe catalysts62. Van der Waals interaction was described by using the DFT-D3 method63. The electron exchange correlation function was described within the generalized gradient approximation (GGA)61 with the Perdew-Burke-Ernzerhof (PBE) functional. The cut-off energy of 450 eV for plane-wave was set for all calculations while the atomic position was fully relaxed until the residual forces was less than 0.02 eV/Å. The Brillouin zone was sampled with a 3 × 3 × 1 k-points based on the Gamma-centered Monkhorst-Pack mesh. A vacuum space exceeding 30 Å was employed to avoid the interaction between two periodic units in z direction. The Fe/Pt 2 × 2 × 1 supercell was built based on Pt (111) orientation crystal where Pt atoms in one layer were replaced by Fe atoms.
The computational hydrogen electrode (CHE) model was used to calculate the free energy of reactions involving electron-proton transfer. According to the method presented by Nørskov64, the Gibbs free energy diagrams for ORR were calculated by the equation:
where ΔE is the thermodynamic energy difference of reactants and products; ΔZPE and ΔS are the energy difference of zero-point energy and entropy; T is the temperature and 298.15 K is employed.
The free energy of H+ ions has been corrected by the concentration dependence of the entropy:
(0.059526 for 0.1 M HClO4; 0.773844 for 0.1 M KOH).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information. Source data are provided as a Source Data file. Source data are provided with this paper.
References
Wang, X. X., Swihart, M. T. & Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2, 578–589 (2019).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Guo, W. et al. A closely packed Pt1.5Ni1-x/Ni-N-C hybrid for relay catalysis towards oxygen reduction. Energy Environ. Sci. 16, 148–156 (2023).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Xiao, F. et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 5, 503–512 (2022).
Zhang, J. et al. Stabilizing pt-based electrocatalysts for oxygen reduction reaction: Fundamental understanding and design strategies. Adv. Mater. 33, e2006494 (2021).
Zhao, X. & Sasaki, K. Advanced Pt-based core-shell electrocatalysts for fuel cell cathodes. Acc. Chem. Res. 55, 1226–1236 (2022).
Tao L. et al. Precise synthetic control of exclusive ligand effect boosts oxygen reduction catalysis. Nat. Commun. 14, 6893 (2023).
Lv, F. et al. A highly efficient atomically thin curved PdIr bimetallene electrocatalyst. Natl Sci. Rev. 8, nwab019 (2021).
Kodama, K., Nagai, T., Kuwaki, A., Jinnouchi, R. & Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 16, 140–147 (2021).
Nie, Y., Li, L. & Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 44, 2168–2201 (2015).
Gan, T. & Wang, D. S. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res. 17, 18–38 (2024).
Zhou, W. et al. Regulating the scaling relationship for high catalytic kinetics and selectivity of the oxygen reduction reaction. Nat. Commun. 13, 6414 (2022).
Gao, R. et al. Pt/Fe2O3 with Pt-Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 6, 614–623 (2021).
Wei, X. et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem. 9, 181–197 (2023).
He, W., Wang, Y., Jiang, C. & Lu, L. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts. Chem. Soc. Rev. 45, 2396–2409 (2016).
Yang, G. et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 12, 1734 (2021).
Liu, M. et al. Concave Pt-Zn nanocubes with high-index faceted Pt skin as highly efficient oxygen reduction catalyst. Adv. Sci. 9, e2200147 (2022).
Liu, S. et al. Atomically dispersed iron sites with a nitrogen-carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 7, 652–663 (2022).
Yang, C. L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Qiao, Z. et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: Performance and durability improvements. Energy Environ. Sci. 14, 4948–4960 (2021).
Huang, L. et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commun. 13, 6703 (2022).
Ao, X. et al. Atomically dispersed Fe-N-C decorated with Pt-alloy core-shell nanoparticles for improved activity and durability towards oxygen reduction. Energy Environ. Sci. 13, 3032–3040 (2020).
Yoo, T. Y. et al. Scalable production of an intermetallic Pt-Co electrocatalyst for high-power proton-exchange-membrane fuel cells. Energy Environ. Sci. 16, 1146–1154 (2023).
Miao, X. et al. Stabilizing single-atomic Pt by forming Pt-Fe bonds for efficient diboration of alkynes. Adv. Mater. 35, e2211790 (2023).
Xiao, M. et al. Engineering energy level of metal center: Ru single-atom site for efficient and durable oxygen reduction catalysis. J. Am. Chem. Soc. 141, 19800–19806 (2019).
Bletsa, E., Solakidou, M., Louloudi, M. & Deligiannakis, Y. Oxidative catalytic evolution of redox- and spin-states of a Fe-phthalocyanine studied by EPR. Chem. Phys. Let. 649, 48–52 (2016).
Zhao, K. M. et al. Insight into the mechanism of axial ligands regulating the catalytic activity of Fe-N4 sites for oxygen reduction reaction. Adv. Energy Mater. 12, 2103588 (2022).
Bartholomew, C. Surface composition and chemistry of supported platinum-iron alloys. J. Catal. 29, 278–291 (1973).
Mehmood, A. et al. High loading of single atomic iron sites in Fe-NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 5, 311–323 (2022).
Li, Z. et al. Tuning the spin density of cobalt single-atom catalysts for efficient oxygen evolution. ACS Nano 15, 7105–7113 (2021).
Yang, X. et al. Unveiling the axial hydroxyl ligand on Fe-N4-C electrocatalysts and its impact on the pH-dependent oxygen reduction activities and poisoning kinetics. Adv. Sci. 7, 2000176 (2020).
Bae, G., Chung, M. W., Ji, S. G., Jaouen, F. & Choi, C. H. pH effect on the H2O2-induced deactivation of Fe-N-C catalysts. ACS Catal. 10, 8485–8495 (2020).
Choi, C. H. et al. The achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 11, 3176–3182 (2018).
Luo, E. et al. Single-atom Cr-N4 sites designed for durable oxygen reduction catalysis in acid media. Angew. Chem. Int. Ed. 58, 12469–12475 (2019).
Mechler, A. K. et al. Stabilization of iron-based fuel cell catalysts by non-catalytic platinum. J. Electrochem Soc. 165, F1084–F1091 (2018).
Nayak, S., McPherson, I. J. & Vincent, K. A. Adsorbed intermediates in oxygen reduction on platinum nanoparticles observed by in situ IR spectroscopy. Angew. Chem. Int. Ed. 57, 12855–12858 (2018).
Xie, Y. et al. Direct oxygen-oxygen cleavage through optimizing interatomic distances in dual single-atom electrocatalysts for efficient oxygen reduction reaction. Angew. Chem. Int. Ed. 62, e202301833 (2023).
Xia, H. et al. Evolution of stabilized 1T‐MoS2 by atomic‐interface engineering of 2H‐MoS2/Fe-Nx towards enhanced sodium ion storage. Angew. Chem. Int. Ed. 62, e202218282 (2023).
Luo, M. & Koper, M. T. M. A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111). Nat. Catal. 5, 615–623 (2022).
Stiles, M. D. Interlayer exchange coupling. J. Magn. Magn. Mater. 200, 322–337 (1999).
An, Y., Duan, L., Liu, T., Wu, Z. & Liu, J. Structural and magnetic properties of Pt in Co/Pt multilayers. Appl Surf. Sci. 257, 7427–7431 (2011).
Zhang, N. et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 3, 509–521 (2020).
Zhao, Z. et al. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 17, 968–975 (2022).
Liu, X. et al. Inducing covalent atomic interaction in intermetallic Pt alloy nanocatalysts for high-performance fuel cells. Angew. Chem. Int. Ed. 62, e202302134 (2023).
Liu, J. et al. Ultrathin nanotube structure for mass-efficient and durable oxygen reduction reaction catalysts in pem fuel cells. J. Am. Chem. Soc. 144, 19106–19114 (2022).
Zhao, Z. et al. Tailoring a three-phase microenvironment for high-performance oxygen reduction reaction in proton exchange membrane fuel cells. Matter 3, 1774–1790 (2020).
Li, J. et al. Hard-magnet l10-CoPt nanoparticles advance fuel cell catalysis. Joule 3, 124–135 (2019).
Liang, J. et al. Biaxial strains mediated oxygen reduction electrocatalysis on fenton reaction resistant l10‐PtZn fuel cell cathode. Adv. Energy Mater. 10, 2000179 (2020).
Chen K. et al. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat. Commun. 11, 4173 (2020).
Yu, L. et al. Disclosing the natures of carbon edges with gradient nanocarbons for electrochemical hydrogen peroxide production. Matter 5, 1909–1923 (2022).
Zhao B. et al. Optimizing electrocatalytic oxygen reduction by adjacent C-O-C structure-driven charge separation on FeN4 active sites. Appl. Catal. B Environ., 324, 122251 (2023).
Xue D. et al. Disentangling the activity‐stability trade‐off of pyrrolic N‐coordinated Fe-N4 catalytic sites for long‐life oxygen reduction reaction in acidic medium. Adv. Energy Mater. 14, 2303733 (2024).
Zhang, H. et al. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 12, 2548–2558 (2019).
Zhang, J. et al. Boosting the performance of the Fe-N-C catalyst for the oxygen reduction reaction by introducing single-walled carbon nanohorns as branches on carbon fibers. J. Mater. Chem. A 7, 23182–23190 (2019).
Liang J. et al. Gas-balancing adsorption strategy towards noble-metal-based nanowire electrocatalysts. Nat. Catal. 7, 719–732 (2024).
Yarlagadda, V. et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 3, 618–621 (2018).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Guđmundsson, A. et al. Efficient formation of 2,3-dihydrofurans via iron-catalyzed cycloisomerization of α-allenols. ACS Catal. 8, 12–16 (2017).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Norskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
This work was financially supported by the Key Projects of the National Natural Science Foundation of China (U22A20107), the Key Projects of the Henan Provincial Science and Technology R&D Program Joint Fund (222301420001), the Distinguished Young Scholars Innovation Team of Zhengzhou University (32320275). Acknowledge the Beijing Synchrotron Radiation Facility (BSRF) 1W1B station for XAS measurements. Li-Rong Zheng is grateful to the support of XAS.
Author information
Authors and Affiliations
Contributions
D.P.X., M.H.S., and J.N.Z. conceived the project. D.P.X. carried out the synthesis, most of the structural characterizations, electrochemical tests, and fuel cell measurements. Y.Y. and Y.F.W. performed the DFT calculations. Y.F.Y. and H.Z.G. performed the operando magnetometry characterizations. S.R.X., J.Q.Z., M.H.S., and J.N.Z. discussed the results and commented on the manuscript. D.P.X. and J.N.Z. analyzed the data and co-wrote the paper. J.N.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, D., Yuan, Y., Yu, Y. et al. Spin occupancy regulation of the Pt d-orbital for a robust low-Pt catalyst towards oxygen reduction. Nat Commun 15, 5990 (2024). https://doi.org/10.1038/s41467-024-50332-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50332-x
This article is cited by
-
Rational design of precatalysts and controlled evolution of catalyst-electrolyte interface for efficient hydrogen production
Nature Communications (2025)
-
High yielding hierarchical porous Fe-N-C with enriched effective active sites: Promoting oxygen reduction reaction electrocatalytic kinetics
Science China Technological Sciences (2025)