Abstract
Molecular structure-editing through nitrogen insertion offers more efficient and ingenious pathways for the synthesis of nitrogen-containing compounds, which could benefit the development of synthetic chemistry, pharmaceutical research, and materials science. Substituted amines, especially nitrogen-containing alkyl heterocyclic compounds, are widely found in nature products and drugs. Generally, accessing these compounds requires multiple steps, which could result in low efficiency. In this work, a molecular editing strategy is used to realize the synthesis of nitrogen-containing compounds using aryl alkanes as starting materials. Using derivatives of O-tosylhydroxylamine as the nitrogen source, this method enables precise nitrogen insertion into the Csp2-Csp3 bond of aryl alkanes. Notably, further synthetic applications demonstrate that this method could be used to prepare bioactive molecules with good efficiency and modify the molecular skeleton of drugs. Furthermore, a plausible reaction mechanism involving the transformation of carbocation and imine intermediates has been proposed based on the results of control experiments.
Similar content being viewed by others
Introduction
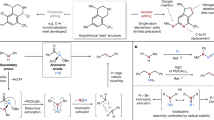
Amines are important organic compounds widely used in organic synthesis, materials science, and pharmaceutical research1,2. It is worth noting that some substituted amines, especially nitrogen-containing alkyl heterocyclic compounds, are key fragments of a variety of biologically active molecules (Fig. 1a)3,4,5,6,7,8. Furthermore, installing nitrogen-containing fragments into bioactive molecules is a key strategy for new drug development9,10. For example, converting the carbonyl group in erythromycin into amino fragments can produce azithromycin, an antibiotic with a wider antibacterial spectrum and better drug metabolism (Fig. 1b)11. However, inserting a nitrogen-containing unit into a carbon framework requires multiple steps with low efficiency, which is usually a challenging task (Fig. 1c)12,13. The synthetic strategy of molecular structure-editing could precisely add, swap, or delete single atom in the molecular skeleton, revolutionizing chemical synthesis route design14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30. The pioneering elegant achievements regarding nitrogen atom insertion into hydrocarbons have well illustrated that aromatic N-containing compounds can be intelligently prepared from easily available starting materials31,32,33,34,35,36,37. Herein, we developed a transition metal-free selective nitrogen insertion into aryl alkanes (Fig. 1d). This chemistry could be used to obtain bioactive molecules with good efficiency and modify the molecular skeleton of drugs.
Aryl amine motifs are prevalent in various valuable compounds, such as drugs, natural products, and functional materials38,39. Therefore, developing practical methods to prepare derivatives of aryl amine is of general interest to the community of synthetic chemistry, drug development, materials science, and various chemical industries40,41. Generally, the methods to construct such important fragments include transition-metal-catalyzed cross-coupling reactions42,43, reductive reactions of azo or nitro compounds44, and direct arene aminations45,46,47,48,49,50. In recent decades, C-C bond-breaking transformations have been developed as powerful tool to construct new chemical bonds51,52,53,54. However, these methods usually convert the methylene unit into a byproduct, which could break the alkane ring (or chain) of the starting material55,56,57,58,59. Consequently, they cannot be used in the synthesis of nitrogen-containing heterocyclic products. In this present work, by leveraging a molecular editing strategy, we developed a method for the precise nitrogen insertion into the Csp2-Csp3 bond of aryl alkanes, which could access substituted amines, especially nitrogen-containing alkyl heterocyclic compounds, with good atom economy (Fig. 1d).
Results
Optimization of the selective nitrogen insertion
The aim of our research is to develop more efficient and applicable synthesis methods for activating carbon-carbon bonds to form carbon-nitrogen bonds60,61,62,63. Based on our previous results regarding C-C bond cleavage amination60,61,62,63, this study commenced with the one-pot and two-steps reaction of compounds 1 and 2 (Table 1). Firstly, the impact of different oxidants on the outcome of the metal-free reaction was investigated (Table 1, entries 1–4). The reaction with DDQ, 1,4-benzoquinone, or chloranil yielded product 3, but using H2O2 as the oxidant did not result in the desired product. Among these oxidants, DDQ was the most effective (Table 1, entry 1). Interestingly, further studies suggested that NaBH4 and HBpin could be used as reluctant at the second step of the reaction to access 3, albeit with lower yields (Table 1, entries 5 and 6). Next, the reaction was conducted in different solvents (Table 1, entries 7–11). Using 2,2,2-trifluoroethanol as the solvent afforded 3 with a 95% yield (Table 1, entry 7). Furthermore, the desired product could be generated with lower yields when using iPrOH, THF, or DCM as solvents (Table 1, entries 8–10). However, the reaction did not proceed when DMSO was used as the solvent (Table 1, entry 11). Moreover, increasing the temperature to 60 °C resulted in a slight decrease in the yield of 3 (Table 1, entry 12). The reaction proceeded at 0 °C, but only produced 3 with a 57% yield (Table 1, entry 13). Further control experiments illustrated that H2O is an important additive for improving the yield (Table 1, entry 14). We speculated that H2O plays a role in adjusting the solubility of the substances and additives. Importantly, without DDQ or NaBH3CN, the present reaction did not work (Table 1, entries 15 and 16). These results suggest that these two reagents are essential for the present reaction.
The investigation of substrate scope
After establishing the optimal reaction conditions, the tolerance of amination reagents was investigated (Fig. 2, 3–17). Generally, substrates with various functional groups could be transformed into the desired product. First, the gram-scale reaction could work well with almost no decrease in the yield of the desired product (Fig. 2, 3). When the methyl group was extended into a longer alkyl chain, the corresponding products could be obtained with a good yield (Fig. 2, 3–5). Interestingly, amination reagents with secondary, tertiary, or benzyl carbon-hydrogen bonds could be used to synthesize the desired product (Fig. 2, 6 to 9). Furthermore, some valuable but active groups, such as ether, ester, and bromine groups, could be smoothly transformed into the desired product from the starting materials (Fig. 2, 10 to 12). Notably, both alkenyl and alkynyl groups could be compatible with this amination reaction. Moreover, both terminal and internal carbon-carbon unsaturated bonds on the precursors could be transferred into the products with good to excellent yield (Fig. 2, 13 to 16). Importantly, in addition to the tertiary amine products mentioned above, the present method could be used to insert secondary amines into the aryl alkanes (Fig. 2, 17). The amination reagents with various valuable functional groups proved to be well compatible with the present chemistry (Fig. 2, 10 to 17), unlike in our previous studies involving amination reagents, where they were ineffective63. Then, the substrate scope regarding aryl alkanes was studied (Fig. 2, 18–45). Our present nitrogen insertion reaction could be used as a powerful tool to synthesize nitrogen-containing alkyl heterocyclic rings, which are key units in various bioactive molecules (Fig. 2, 18 to 29). First, besides the bibenzyl position (Fig. 2, 3 and 18), substrates with normal benzyl groups could be smoothly converted into the desired product (Fig. 2, 19 to 26). Further studies suggested that functional groups, such as methoxy, bromo and phenyl groups, on the benzene ring could be tolerated (Fig. 2, 21–26). Interestingly, in all of asymmetric examples, the reported products could be generated without producing any isomeric byproduct. For products 21, 23 and 25, the -OMe, -Br, and -Ph groups at the para-position could facilitate the generation of benzyl carbocation, leading to the para-nitrogen insertion product. Conversely, the -Br and -Ph groups at the ortho-position would shield the benzyl site, favoring meta-product formation (Fig. 2, 24 and 26). The ortho-OMe group could promote the generation of ortho-benzyl carbocation without covering the benzyl site, allowing for the formation of product 22 as the ortho-insertion product. To our delight, oxygen-heterocyclic starting material could afford nitrogen insertion product with 86% yield (Fig. 2, 27). Importantly, the present method could be used to construct 8-membered and 6-membered nitrogen-heterocyclic rings from readily available starting materials (Fig. 2, 28 and 29). We next investigated the scope of noncyclic aryl alkanes under the optimal reaction conditions (Fig. 2, 30–45). The results demonstrated both secondary and tertiary benzyl alkanes could be compatible with the nitrogen insertion reaction. Further scoping illustrated that valuable functional groups on the phenyl ring, such as -OMe, -Br, -NH2, -NHTs and -Bpin, could be transferred into the desired products (Fig. 2, 35–41). Interestingly, when double or triple -iPr groups were installed on the phenyl ring, the present reaction could produce mono-amination products with great yield (Fig. 2, 42 and 43). In addition to phenyl alkanes, other types of aryl alkanes, such as 2-ethylnaphthalene and 5-isopropylbenzofuran, could be efficiently converted into desired products (Fig. 2, 44 and 45). Unfortunately, toluene and p-isopropyl benzonitrile cannot be converted into target products (Fig. 2, 46 and 47).
Aryl alkanes (0.3 mmol), amination reagent TsONHR (0.45 mmol), DDQ (0.45 mmol), H2O (3.0 mmol), HFIP (1.5 mL), N2, 25 °C, 12 h, then NaBH3CN (1.5 mmol), 2 h, isolated yield. aAryl alkanes (5.0 mmol), amination reagent TsONHR (7.5 mmol), DDQ (7.5 mmol), H2O (50.0 mmol), HFIP (25.0 mL), N2, 25 °C, 12 h, then NaBH3CN (25.0 mmol), 2 h. b60 °C, 24 h instead of 25 °C, 12 h, then NaBH3CN (25.0 mmol), 2 h. c60 h instead of 12 h, then NaBH3CN (25.0 mmol), 2 h.
The studies of synthetic application
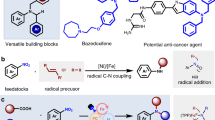
As the insertion of nitrogen atoms into biologically active molecules is an important strategy in drug development, the modification of various bioactive molecules using our present transformation has been investigated herein. Interestingly, nitrogen could be smoothly inserted into the derivatives of ibuprofen and dehydroabietic acid (Fig. 3a, 48 and 49). The results of compound 48 suggest that the benzyl C-H bond with an electron-withdrawing group is unfavorable, while the data from compound 49 illustrate that in this example the tertiary benzyl C-H bond on the carbon chain is much more favorable than secondary benzyl C-H bond on the carbon ring motif. Importantly, this nitrogen insertion reaction could be used to prepare nonpeptidergic inhibitors of the human cytomegalovirus-encoded chemokine receptor in a practical manner (Fig. 3b). Under the optimal reaction conditions, the compound 50 could be converted into the key intermediate 51 with a 70% yield, and further substitution reaction with 52 could afford the desired product 54. Compared with the previous method, our synthetic strategy uses cheaper starting materials, avoids inconvenient operations such as employing butyl lithium and microwave under high temperature, and provide higher yields with fewer operation steps64,65. The results in Fig. 3 illustrate the great application prospects of our developed synthetic methodology.
Reaction mechanism study
To investigate the reaction mechanism, some control experiments have been designed (Fig. 4). Firstly, the source of hydrogen atoms in the product was investigated. Interestingly, when deuterated starting material (55-D1) or deuterated water was used, the non-deuterated product could be isolated with a good yield (Fig. 4a, 1 and 2). Importantly, if NaBD4 was employed, the deuterated product (D% = 95%) could be isolated with a 90% yield (Fig. 4a, 3). These data could well demonstrate that the new hydrogen atom in the product originated from the reductant. Further kinetic isotope studies afforded a KIE value of 5.3:1, which supports the idea that C-H bond breaking is the selectivity-determining step (Fig. 4b). Moreover, a crossover experiment using a mixture of 57 and 55 as starting materials afforded 30 and 35 as products without the generation of 58 and 31 (Fig. 4c). These results imply that the reaction mechanism of Csp2-Csp3 bond cleavage and nitrogen insertion is an intramolecular reaction. To further investigate the effect of different substituents, competition experiments involving various phenyl alkanes were designed (Fig. 4d). Firstly, the reaction using an equal mixture of pentylbenzene and cyclohexylbenzene yielded 23% N-cyclohexyl-N-methylaniline with trace amounts of N-methyl-N-pentylaniline, suggesting that tertiary C-H bonds are more favorable than secondary C-H bonds in the present reaction. Secondly, the reaction using an equal mixture of isopropyl benzene and 1-isopropyl-4-methoxybenzene afforded 92% of N-isopropyl-4-methoxy-N-methylaniline with trace N-isopropyl-N-methylaniline, demonstrating that the presence of electron-donating substituents in the aromatic ring could enhance the reaction. Then, TEMPO was used to trap the radical intermediate in the reaction system. However, the data illustrated that TEMPO did not affect the reaction, which afforded 83% of 3 and recovered 87% TEMPO (Fig. 4e). This data suggested that the present transformation does not proceed through a radical reaction process. Based on the above studies, the possible reaction mechanisum has been proposed (Fig. 4f). First, carbocation 62 could be generated through the oxidation reaction between starting material 1 and DDQ or a nitrogen radical, which is produced from the reaction of DDQ and TsONHMe (Please see the Supplementary Figs 46–48 for more details)61,62. Then, the reaction of the animation reagent and 62 could afford the key intermediate 6357,66. The following rearrangement could produce the imine intermediate 6467. Then, further reduction could afford compound 3 as the final product.
Discussion
In conclusion, we have developed a reaction for realizing nitrogen insertion into aryl alkanes. By employing a molecular editing strategy, this chemistry can expand the ring or lengthen the carbon chain under ambient reaction conditions. Notably, this chemistry, which is compatible with various functional groups, has been successfully used to modify drug molecules and synthesize bioactive compounds. Furthermore, the possible reaction mechanism has been proposed based on the results of radical trapping, deuterium labeling and isotope competition experiments. Further studies for synthetic applications are ongoing in our laboratory.
Methods
In a 25 mL Schlenk tube that had been oven-dried and contained a stirring bar, the aminating reagent (0.45 mmol, 1.5 equiv.) and DDQ (0.45 mmol, 1.5 equiv.) were charged. The tube was then evacuated and back-filled under N2 flow (this sequence was repeated three times). Water (3.0 mmol, 10.0 equiv.), aryl alkanes (0.3 mmol, 1.0 equiv.), and HFIP (1.5 mL) were added, then the mixture was stirred at room temperature for 12 h. Subsequently, NaBH3CN (1.5 mmol, 5.0 equiv.) was added to the reaction mixture and stirred for 2 h at room temperature. The reaction was quenched with 2.0 mL saturated NaHCO3 aq. and 3.0 mL H2O. The mixture was then extracted with DCM (3.0 mL × 3), and the combined organic layers were dried over Na2SO4, filtered, and concentrated by rotary evaporation. The residue was purified by silica gel chromatography (EtOAc/petroleum ether) to afford the desired product.
Data availability
Supplementary information and chemical compound information accompany this paper at www.nature.com/ncomms. The data supporting the results of this work are included in this paper or in the Supplementary Information and are also available upon request from the corresponding author.
References
Trowbridge, A. et al. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Murugesan, K. et al. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 49, 6273–6328 (2020).
Ma, D. et al. Synthesis of 7,8-Disubstituted Benzolactam-V8 and its binding to protein Kinase C. Bioorg. Med. Chem. Lett. 11, 99–101 (2021).
Duan, Z. et al. Antitumor activity of Mianserin (a Tetracyclic Antidepressant) primarily driven by the inhibition of SLC1A5‑Mediated glutamine transport. Investig. New Drugs 40, 977–989 (2022).
Ghali, J. K. et al. Tolvaptan. Nat. Rev. Drug. Discov. 8, 611–612 (2009).
Dai, X. et al. Metabolomics-based study on the discriminative classification models and toxicological mechanism of estazolam fatal intoxication. Metabolites 13, 567–587 (2023).
Pritchett, B. P., Kikuchi, J., Numajiri, Y. & Stoltz, B. M. Enantioselective Pd-catalyzed allylic alkylation reactions of Dihydropyrido[1,2-a]indolone substrates: efficient syntheses of (-)-Goniomitine, (+)-Aspidospermidine, and (-)-Quebrachamine. Angew. Chem. Int. Ed. 55, 13529–13532 (2016).
Miksa, B. et al. Chlorambucil labelled with the Phenosafranin Scaffold as a new chemotherapeutic for imaging and cancer treatment. Colloids Surf. B 159, 820–828 (2017).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, 244–252 (2019).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Lee, Y. et al. Chemistry and biology of macrolide antiparasitic agents. J. Med. Chem. 54, 2792–2804 (2011).
Stevenson, P. J. Cyclic arylamines. Sci. Synth. 31b, 1885–1938 (2007).
Ciganek, E. Electrophilic amination of carbanions, enolates, and their surrogates. Org. React. 72, 1–366 (2008).
Peplow, M. ‘Almost magical’: chemists can now move single atoms in and out of a molecule’s core. Nature 618, 21–24 (2023).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Cheng, Q. et al. Skeletal editing of pyridines through atom-pair swap from CN to CC. Nat. Chem. https://doi.org/10.1038/s41557-023-01428-2 (2024).
Kim, S. F. et al. Wavelength-dependent reactivity, and expanded reactivity of N-Aryl Azacycle photomediated ring contractions. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.3c13982 (2024).
Wu, F.-P. et al. Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion. Nat. Catal. https://doi.org/10.1038/s41929-023-01089-x (2024).
Schmitt, H. L. et al. Regiodivergent ring-expansion of oxindoles to quinolinones. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.3c12119 (2024).
Wright, B. A. et al. Skeletal editing approach to bridge-functionalized Bicyclo[1.1.1]pentanes from Azabicyclo[2.1.1]hexanes. J. Am. Chem. Soc. 145, 10960–10966 (2023).
Bartholomew, G. L., Carpaneto, F. & Sarpong, R. Skeletal editing of pyrimidines to pyrazoles by formal carbon deletion. J. Am. Chem. Soc. 144, 22309–22315 (2022).
Hyland, E. E., Kelly, P. Q., McKillop, A. M., Dherange, B. D. & Levin, M. D. Unified access to pyrimidines and quinazolines enabled by N-N cleaving carbon atom insertion. J. Am. Chem. Soc. 144, 19258–19264 (2022).
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Qin, H. et al. N-atom deletion in nitrogen heterocycles. Angew. Chem. Int. Ed. 60, 20678–20683 (2021).
Hui, C., Brieger, L., Strohmann, C. & Antonchick, A. P. Stereoselective synthesis of cyclobutanes by contraction of pyrrolidines. J. Am. Chem. Soc. 143, 18864–18870 (2021).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Dherange, B. D., Kelly, P. Q., Liles, J. P., Sigman, M. S. & Levin, M. D. Carbon atom insertion into pyrroles and indoles promoted by chlorodiazirines. J. Am. Chem. Soc. 143, 11337–11344 (2021).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Mykura, R. et al. Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes. Nat. Chem. 16, 771–779 (2024).
Woo, J., Stein, C., Christian, A. H. & Levin, M. D. Carbon-to-nitrogen single-atom transmutation of azaarenes. Nature 623, 77–82 (2023).
Li, H. et al. Rhodium-catalyzed intramolecular nitrogen atom insertion into arene rings. J. Am. Chem. Soc. 145, 17570–17576 (2023).
Liu, S. & Cheng, X. Insertion of ammonia into alkenes to build aromatic N-heterocycles. Nat. Commun. 13, 425–432 (2022).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Patel, S. C. & Burns, N. Z. Conversion of aryl azides to aminopyridines. J. Am. Chem. Soc. 144, 17797–17802 (2022).
Wang, J., Lu, H., He, Y., Jing, C. & Wei, H. Cobalt-catalyzed nitrogen atom insertion in arylcycloalkenes. J. Am. Chem. Soc. 144, 22433–22439 (2022).
Becker, O. M. et al. An integrated in silico 3D model-driven discovery of a novel, potent, and selective Amidosulfonamide 5-HT1A Agonist (PRX-00023) for the treatment of anxiety and depression. J. Med. Chem. 49, 3116–3135 (2006).
Ekins, S., Balakin, K. V., Savchuk, N. & Ivanenkov, Y. Insights for human ether-a-Go-Go-Related gene potassium channel inhibition using recursive partitioning and Kohonen and Sammon mapping techniques. J. Med. Chem. 49, 5059–5071 (2006).
Kärkäs, M. D. Electrochemical strategies for C-H functionalization and C-N bond formation. Chem. Soc. Rev. 47, 5786–5865 (2018).
Zhao, Y. & Xia, W. Recent advances in radical-based C-N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 47, 2591–2608 (2018).
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534–1544 (2018).
Surry, D. S. & Buchwald, S. L. Biaryl phosphane ligands in palladium-catalyzed amination. Angew. Chem. Int. Ed. 47, 6338–6361 (2008).
Hartwig, J. F., Shekhar, S., Shen, Q. & Barrios-Landeros, F. Synthesis of anilines. ChemInform 38, 455–536 (2007).
Shin, K., Kim, H. & Chang, S. Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C-H amination. Acc. Chem. Res. 48, 1040–1052 (2015).
Paudyal, M. P. et al. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Park, Y., Park, K. T., Kim, J. G. & Chang, S. Mechanistic studies on the Rh(III)-mediated amido transfer process leading to robust C–H amination with a new type of amidating reagent. J. Am. Chem. Soc. 137, 4534–4542 (2015).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective Arene C-H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Shrestha, R., Mukherjee, P., Tan, Y., Litman, Z. C. & Hartwig, J. F. Sterically controlled, palladium-catalyzed intermolecular amination of arenes. J. Am. Chem. Soc. 135, 8480–8483 (2013).
Tsang, W. C. P., Zheng, N. & Buchwald, S. L. Combined C-H functionalization/C-N bond formation route to carbazoles. J. Am. Chem. Soc. 127, 14560–14561 (2005).
Liang, Y.-F. et al. Carbon-carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
Song, F., Guo, T., Wang, B.-Q. & Shi, Z.-J. Catalytic activations of unstrained C-C bond involving organometallic intermediates. Chem. Soc. Rev. 47, 7078–7115 (2018).
Kim, D.-S., Park, W.-J. & Jun, C.-H. Metal-organic cooperative catalysis in C-H and C-C bond activation. Chem. Rev. 117, 8977–9015 (2017).
Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C-C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017).
He, Z., Moreno, J. A., Swain, M., Wu, J. & Kwon, O. Aminodealkenylation: ozonolysis and copper catalysis convert C(sp3)-C(sp2) Bonds to C(sp3)-N bonds. Science 381, 877–886 (2023).
Lv, X.-Y., Abrams, R. & Martin, R. Copper-Catalyzed C(sp3)-amination of Ketone-derived Dihydroquinazolinones by Aromatization-driven C-C bond scission. Angew. Chem. Int. Ed. 62, e202217386 (2023).
Anugu, R. R. & Falck, J. R. Site-selective amination and/or nitrilation via metal-free C(sp2)-C(sp3) cleavage of benzylic and allylic alcohols. Chem. Sci. 13, 4821–4827 (2022).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C-N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Mao, R., Frey, A., Balon, J. & Hu, X. Decarboxylative C(sp3)-N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018).
Liu, J. et al. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles. Science 367, 281–285 (2020).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon-carbon amination. Nat. Chem. 11, 71–77 (2019).
Qin, C., Zhou, W., Chen, F., Ou, Y. & Jiao, N. Iron-catalyzed C-H and C-C bond cleavage: a direct approach to amides from simple hydrocarbons. Angew. Chem. Int. Ed. 50, 12595–12599 (2011).
Niu, C. et al. Selective ring-opening amination of isochromans and tetrahydroisoquinolines. Angew. Chem. Int. Ed. 63, https://doi.org/10.1002/anie.202401318 (2024).
Vischer, H. F. et al. Identification of Novel Allosteric Nonpeptidergic Iinhibitors of the Human Cytomegalovirus-encoded Chemokine Receptor US28. Bioorg. Med. Chem. 18, 675–688 (2010).
Prices refer to Combi-Blocks. The website of the Combi-Blocks is: https://www.combi-blocks.com.
Wang, T. et al. Hydroxylamine-mediated C-C Amination via an Aza-hock Rearrangement. Nat. Commun. 12, 7029–7039 (2021).
Sandvoß, A. & Wahl, J. M. From cycloalkanols to heterocycles via nitrogen insertion. Org. Lett. 25, 5795–5799 (2023).
Acknowledgements
We acknowledge the National Key R&D Program of China (No.2021YFA1501700), the NSFC (No. 22371203, 22131002, 22071005, and 22161142019), the Beijing Nova Program (No. Z201100006820099), and the Tencent Foundation for financial support.
Author information
Authors and Affiliations
Contributions
N. Jiao and C. Zhang conceived the research. Z. Zhang and Q. Li carried out experiments. Z. Zhang, Z. Cheng, N. Jiao, and C. Zhang analyzed results. N. Jiao and C. Zhang wrote the manuscript with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Li, Q., Cheng, Z. et al. Selective nitrogen insertion into aryl alkanes. Nat Commun 15, 6016 (2024). https://doi.org/10.1038/s41467-024-50383-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50383-0
This article is cited by
-
Divergent synthesis of N heterocycles from carbocycles enabled by electrochemical nitrogen atom insertion
Nature Synthesis (2025)
-
Precise control of selective nitrogen atom insertion into five-membered cyclic β-ketoesters
Nature Communications (2025)