Abstract
In zebrafish, brain lymphatic endothelial cells (BLECs) are essential for meningeal angiogenesis and cerebrovascular regeneration. Although epidermal growth factor-like domain 7 (Egfl7) has been reported to act as a pro-angiogenic factor, its roles in lymphangiogenesis remain unclear. Here, we show that Egfl7 is expressed in both blood and lymphatic endothelial cells. We generate an egfl7 cq180 mutant with a 13-bp-deletion in exon 3 leading to reduced expression of Egfl7. The egfl7 cq180 mutant zebrafish exhibit defective formation of BLEC bilateral loop-like structures, although trunk and facial lymphatic development remains unaffected. Moreover, while the egfl7 cq180 mutant displays normal BLEC lineage specification, the migration and proliferation of these cells are impaired. Additionally, we identify integrin αvβ3 as the receptor for Egfl7. αvβ3 is expressed in the CVP and sprouting BLECs, and blocking this integrin inhibits the formation of BLEC bilateral loop-like structures. Thus, this study identifies a role for Egfl7 in BLEC development that is mediated through the integrin αvβ3.
Similar content being viewed by others
Introduction
The lymphatic vessels, including the meningeal lymphatic vessels, play physiological roles in fluid homeostasis, fat and macromolecule absorption, drainage of waste, and immune surveillance1. In vertebrates, the lymphatic endothelial cell (LEC) specification and maintenance depend on Prox12,3, and embryonic lymphangiogenesis is driven by VEGFC signaling via VEGFR34. The matrix protein Ccbe1 acts with metalloprotease Adamts3 to generate mature and biologically active VEGF-C protein5,6,7. Integrin β1 is another receptor expressed by LECs that contributes to lymphangiogenesis, and it can modulate the intracellular phosphorylation and activation of VEGFR3 kinase in response to the mechanical stimulus8,9.
Zebrafish is an excellent model for high-resolution, in vivo live imaging of lymphangiogenesis10,11. The trunk lymphangioblasts first sprout from posterior cardinal veins (PCV) and migrate to the horizontal myoseptum (HM). These parachordal lymphangioblasts (PL) then migrate ventrally and dorsally to remodel into the thoracic duct (TD) and the dorsal longitudinal lymphatic vessel (DLLV)12. The facial lymphatic sprout (FLS) differs from the previously characterized trunk lymphatics. It arises from the common cardinal vein (CCV) and other non-venous progenitors13, then migrates to form the lateral facial lymphatic vessel (LFL), the otolithic lymphatic vessel (OLV), the medial facial lymphatic (MFL), and the lymphatic branchial arches (LAAs)14.
Zebrafish also harbour brain lymphatic endothelial cells (BLECs) also known as fluorescent granular perithelial cells (FGPs) or meningeal mural lymphatic endothelial cells (muLECs). These cells show the same gene expression signature as other LECs but do not form vessels. Instead, they form a network of individual cells covering the brain surface15,16,17. This is different from meningeal lymphatic vessels, which attach to the inner surface of the skull18. These BLECs are derived from the choroidal vascular plexus (CVP) at 56 h post-fertilization (hpf) and migrate along the mesencephalic vein (MsV) to form a bilateral loop over the optic tectum at 5 days post-fertilization (dpf). Moreover, the BLECs are crucial in regulating meningeal angiogenesis and managing severe pathological processes like ischemic stroke. After brain vascular injury, BLECs formed lumenized lymphatic vessels and grew into the parenchyma, directed by Cxcl12b/Cxcr4a19. These ingrown lymphatic vessels (iLVs), on the one hand, drain interstitial fluid to resolve edema; on the other hand, they transdifferentiate into early-regenerated blood vessels and act as a “growing track” for nascent blood vessels20,21. Consistent with their lymphatic nature, the development of BLECs depends on the Vegfc/Vegfd/Vegfr3/Ccbe1 signaling pathway. However, whether some new molecular mechanisms regulate BLECs forming a vessel-like loop during the initial sprouting is still unknown.
EGFL7 is a highly conserved secreted angiogenic factor found in vertebrates. This extracellular matrix-bound factor is expressed almost exclusively by endothelial cells and has a specific role in blood vessel development by influencing the extra cellular matrix (ECM) environment22. EGFL7 consists of various putative protein domains, including an elastin microfibril interface (EMI) part and two centrally located EGF-like domains23. It is a specific ligand for integrin on endothelial cells and is strongly associated with fibronectin in the ECM24. Several studies have shown that knockdown of egfl7 in zebrafish, frogs, and HUVECs suppresses endothelial cell proliferation, adhesion, migration, and vascular tube formation25,26,27. However, the egfl7 mutant of zebrafish and one Egfl7-specific knockout mouse line lack obvious phenotypes, which can be explained by activation of compensatory genes or the biological effects of miR-12628,29. EGFL7 also plays a role in vascular repair, CNS inflammation, tumor metastasis, and cancer angiogenesis30,31,32,33. However, it remains unclear whether it plays a role in lymphangiogenesis despite its critical role in angiogenesis.
In this study, we aim to determine whether Egfl7 is also important for BLEC development in zebrafish. We find that Egfl7 plays a role in the early stages of lymphangiogenesis by forming a vessel-like loop via migration and proliferation in the brain, either autonomously or non-autonomously. However, it is not necessary for the specification of BLECs. Although BLECs sprouting depends on Vegfc, Vegfd, and Ccbe1 signaling, Vegfc overexpression hardly rescues the devoid of BLECs caused by egfl7 mutation. Integrin αvβ3 is a specific receptor of EGFL7 found in the CVP and departing BLECs. We observed that the inhibition of integrin αvβ3 impaired BLECs migration. Furthermore, the suppression of integrin-linked kinase (ILK), which regulates VEGFR3 signaling by controlling its interaction with integrin, partially rescues the complete loss of lymphatic loops observed following egfl7 depletion. Our findings suggest that Egfl7 plays a role in regulating brain lymphatic development through integrin αvβ3.
Results
Egfl7 is required for BLEC formation and brain vascular regeneration
Given that Egfl7 is a critical secreted pro-angiogenic factor and is nearly restricted to the endothelial cells during embryogenesis as well as physiologic and pathologic angiogenesis, we examined whether Egfl7 plays roles in brain vascular regeneration. In the Tg(kdrl: DenNTR) transgenic nitroreductase (NTR)-metronidazole (Mtz) cerebrovascular injury model, BLECs were activated in response to the injuries and quickly ingrew into the injured parenchyma to form lumenized LVs20. Using whole-mount in situ hybridization and fluorescent in situ hybridization (FISH) combined with antibody staining, we found expression of egfl7 in all the endothelium, including BLECs, iLVs, and blood vascular endothelial cells (BECs), regardless of injuries or not (Supplementary Fig. 1). Furthermore, the Tg(egfl7:YFP)cq181 transgenic line was generated to confirm the expression of egfl7 in the endothelium of brain and trunk (Fig. 1). These data suggest the expression of egfl7 in both lymphatic and blood vessels.
a–d Tg(egfl7:YFP) was generated showing YFP in blood vessels and lymphatics. The distribution of YFP is similar with CFP and DsRed in the triple transgenic line Tg(egfl7:YFP; lyve1b: DsRed; kdrl: CFP-NTR) at 5 dpf. n = 25/25 embryos. The low-magnified image shows the dorsal view of the head (a), the high-magnified image in another embryo shows the magnified BLECs loop in the dorsal brain (b). The lateral facial lymphatics of the head shows in c. The trunk lymphatics shows in d. BLECs, brain lymphatic endothelial cells, MsV, mesencephalic vein, OLV, otolithic lymphatic vessel, FL, facial lymphatics, TD, thoracic duct. Scale bar, 50 μm. e Schematic diagram showing the dorsal view of a dissected adult zebrafish brain. f egfl7 promoter-driven YFP is expressed in all BLECs and blood vessels. n = 6/8 adults. g High-magnification inset showing the YFP overlap with DsRed in the double transgenic line Tg(egfl7:YFP; lyve1b: DsRed). Scale bars, 200 μm in f and 50 μm in g.
We next examined lymphatic development and brain vascular regeneration in the egfl7 mutant. The egfl7 cq180 mutant was generated by CRISPR/Cas9, with a 13-bp-deletion in the exon 3, leading to truncation of the EMI domain and a frameshift from amino acid 51 thereby a premature stop codon at amino acid 71 (Supplementary Fig. 2a). This mutation caused nonsense decay of egfl7 mRNA and absence of protein expression (Supplementary Fig. 2b, c). It was similar to the egfl7s981, a well-established mutant allele. Unlike egfl7 morphants that exhibit severe vascular tube formation, egfl7s981 displays normal gross morphologies in vasculogenesis and angiogenesis29. This egfl7 cq180 mutant exhibited no obvious abnormalities in blood vessel development, but the lyve1b + BLECs failed to populate into the bilateral loop-like structures from 4dpf to 6dpf (Fig. 2a–c). To confirm the defective BLEC development, the expression of lymphatic markers including vegfr3, mrc1a, and prox1, was analyzed. Downregulations of vegfr3 and mrc1a were detected in the brain of the mutant (Fig. 2f–j). And the prox1a promoter-driven transgenes34 validated the lack of BLEC bilateral loop-like structures in the mutant (Supplementary Fig. 3a–d). In addition, we examine whether the devoid of BLEC in the mutant could cause defects in brain vascular regeneration. After cerebrovascular injury, the iLV ingrowth and nascent BV regeneration did not occur in the egfl7 cq180 (Supplementary Fig. 3e, f). Consequently, the majority of injured mutants experienced brain edema and died within ten days after Mtz treatment (Supplementary Fig. 3g, h).
a Confocal images of the BLECs, facial lymphatics, and trunk lymphatics in Tg(kdrl: DenNTR;lyve1b: DsRed) transgenic lines from 4 dpf to 6 dpf in WT. b Loss of egfl7 prevents the formation of the BLECs from 4 dpf to 6 dpf, but the facial lymphatics of the lateral head and trunk lymphatics are normal in egfl7 mutant embryos at 6 dpf. c Percentage of embryos that have double lymphatic loops, single loops, and none of the loops in the brain (WT, n = 60 embryos, egfl7-/-, n = 75 embryos, χ2 test). d The statistics show the percentage of TD formation per 6 somites (n = 10 embryos, two-tailed unpaired t-test; ns, no significance. Data are represented as mean ± SD). e The statistics show the total lengths of LFLs, OLVs, and MFLs at 6 dpf (n = 8 embryos, 2way ANOVA multiple comparisons test; ns, no significance. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker)). BLECs, brain lymphatic endothelial cells; LFL lateral facial lymphatic, MFL medial facial lymphatic, LAA lymphatic branchial arches, OLV otolithic lymphatic vessel, ISLV intersegmental lymphatic vessels, DLLV dorsal longitudinal lymphatic vessel, TD thoracic duct. f, g Double labeling of FISH-vegfr3 and anti-DsRed in Tg(lyve1b:DsRed) transgenic background at 6 dpf. Arrowheads point to the BLECs in the top layer of the brain. h, i Double labeling of FISH-mrc1a and anti-Dendra2 in Tg(kdrl:DenNTR) transgenic background at 5 dpf. j The statistics show the number of vegfr3+ and mrc1a + BLECs in WT and egfl7 mutant (n = 9 embryos, 2-way ANOVA multiple comparisons test. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker). k–n Dissection and whole-mount images of BLECs and meningeal vascular in Tg(kdrl: DenNTR;lyve1b: DsRed) adult brain at 6 mpf (months post-fertilization) in WT (k, l) and egfl7 mutant (m, n). o, p The statistics show the number of lyve1b + BLECs in the adult brain (o, n = 6 brains; two-tailed unpaired t test) and the number of BV junctions per 0.16 mm2 of a brain (p, n = 6 brain areas; two-tailed unpaired t test). Data are represented as mean ± SD. Scale bar, 50 μm.
Although the egfl7 cq180 displayed delayed, transient, mild reduction of lymphatic sprouts in OLV, MFL, LFL, and PLs before 4 dpf (Supplementary Fig. 4a–h), the facial lymphatics, TD, and DLLV became recovered by 6 dpf (Fig. 2a, b, d, e). Furthermore, the egfl7 cq180 adults failed to form regular meningeal blood vessel network, which might be caused by the near absence of the lyve1b + BLECs (Fig. 2k–p). Despite that, the egfl7 cq180 adults exhibited overall normal morphologies, and became viable and fertile (Supplementary Fig. 4i). These data indicate that egfl7 is required for the BLEC development, but not for the development of facial and trunk lymphatics. Furthermore, BLECs are not necessary for the viability and fertility of zebrafish.
The mir-126b is located in the intron 6 of egfl7, and has been implicated in lymphatic development and vascular integrity28,35,36. To investigate whether the lack of BLEC and defects in blood vascular regeneration were attributed to the loss of mir-126 function, we analyzed the expression levels of mir-126. Real-time RT-PCRs of WT and egfl7 cq180 demonstrate comparable mir-126 expression levels (Supplementary Fig. 4j), indicating that the BLEC phenotypes in the egfl7 mutant are independent of mir-126.
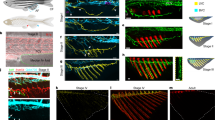
Egfl7 expressed in both LECs and BECs. To test the roles of Egfl7 in BLEC development is cell autonomous or not, we generated two stable transgenic lines Tg(kdrl:egfl7-p2A-GFP)cq182 and Tg(lyve1b:egfl7-p2A-GFP)cq183 under the mutant background to specifically replenish Egfl7 in the BECs and LECs, respectively. In contrast to the Tg(kdrl: GFP) or Tg(lyve1b: GFP) control group, replenishing Egfl7 in either BECs or LECs could rescue the formation of BLEC bilateral loop-like structures in egfl7 cq180 mutants at 6 dpf (Fig. 3b, d, e, g, i, j). These results indicate that both the BEC-derived and LEC-derived Egfl7 are functional for BLEC formation. Additionally, overexpression of Egfl7 in the wild-type BECs using the Tg(kdrl:egfl7-p2A-GFP) transgene induced ectopic BLEC formation in contrast to overexpression of GFP using the Tg(kdrl: GFP) transgene (Fig. 3a, c, e). Whereas Egfl7 overexpression in the wile-type LECs is ineffective to the number of BLECs (Fig. 3f, h, j). This should be caused by more abundant meningeal blood vessels than BLECs in the top layer, so more BLECs were induced by the blood vessel-derived Egfl7. All these data suggest that the endothelial cells-derived Egfl7 is required for the BLEC development.
a–d In the stable transgenic line Tg(kdrl:egfl7-p2A-GFP), the replenishment of Egfl7 in BVs can rescue the absence of BLECs in the egfl7 mutant (d) in contrast to the mutant under the Tg(kdrl: GFP) transgenic line (b). For WT, overexpression of Egfl7 in the BVs results in increased BLECs emerge in the bilateral loop over the brain (c) compared to the WT under the Tg(kdrl:GFP) (a). e The statistics show the number of BLECs per double loops in WT and egfl7 mutant under Tg(kdrl: GFP) and Tg(kdrl:egfl7-p2A-GFP) transgenic lines (n = 24 embryos, 2-way ANOVA. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker)). f–i In contrast to the mutant under Tg(lyve1b: GFP) transgenic line (g), the replenishment of Egfl7 in LECs under Tg(lyve1b:egfl7-p2A-GFP) is able to re-form the BLECs bilateral loop in the egfl7 mutant (i). And overexpression of Egfl7 in WT lymphatics shows the BLECs are unaltered (f, h). j The statistics show the number of BLECs per double loops in WT and egfl7 mutant under Tg(lyve1b: GFP) and Tg(lyve1b:egfl7-p2A-GFP) transgenic lines (n = 24 embryos, 2-way ANOVA. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker)). Scale bar, 50 μm.
Egfl7 is dispensable for specification but necessary for migration and proliferation of BLECs
From about 56 hpf, the vegfr3-positive and low-level kdrl-expressing BLEC progenitors sprout from the CVP, then proliferate and migrate along the MsV to form bilateral loop-like structures of mural lymphatic cells over the brain surface at 3 dpf 37. The BLEC progenitors also express the lymphatic marker prox115. To investigate whether the BLEC progenitors could be specified from the CVP in the egfl7 cq180, we examined Prox1, vegfr3, and mrc1a expression as the markers of LEC fate. We performed anti-Prox1 antibody staining in the lyve1b reporter line, in which the Prox1-positive nuclei were detectable in the lymphatic sprouts from CVP in the egfl7 cq180 at 3 dpf (Fig. 4a–d). Furthermore, combination of FISH and antibody staining indicated that the lyve1b+kdrllow BLEC progenitors in the mutant co-expressed vegfr3 and mrc1a (Fig. 4e–i, arrowheads). However, although the induction of lymphatic identity maintained at the CVP of egfl7 cq180, the number of vegfr3+ and mrc1a + BLECs significantly reduced (Fig. 4j).
a Schematic diagram showing the lateral view and the lower middle layer of the brain, respectively. Black frames indicate the image area of corresponding panels. CVP, choroidal vascular plexus. b, c Immunofluorescence staining for blood endothelial cells (Anti-Dendra2), BLECs (Anti-DsRed), and Anti-Prox1 (blue nuclei) in the WT (b) and egfl7 mutant (c) at 3 dpf. Arrowheads of the magnified 2D single slice image point to the Prox1+ BLECs progenitors sprout from CVP. d The statistics show the ratio of BLECs progenitors positive for Prox1 in WT and egfl7 mutant (n = 9 embryos; two-tailed unpaired t test; ns, no significance. Data are represented as mean ± SD). e–h Confocal image showing the triple labeling of FISH-vegfr3 or FISH-mrc1a, anti-DsRed, and Dendra2 in Tg(kdrl:DenNTR; lyve1b:DsRed) transgenic background in WT (e, g) and egfl7 mutant (f, h). Arrowheads in the magnified image shows vegfr3 mRNA and mrc1a mRNA are expressed in the lyve1b positive and low-level kdrl expressing BLECs progenitors departing the CVP. i The statistics show the ratio of BLECs positive for vegfr3 and mrc1a in WT and egfl7 mutant (n = 8 embryos; 2way ANOVA multiple comparisons test; ns, no significance. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker)). j The statistics show the number of vegfr3+ and mrc1a + BLECs in WT and egfl7 mutant (n = 8 embryos; 2way ANOVA multiple comparisons test. Box plots show the five-number summary of a set of data: including the minimum score (shown at the end of the lower whisker), first (lower) quartile, median, third (upper) quartile, and maximum score (shown at the end of the upper whisker)). Scale bar, 50 μm.
Because BLEC specification was unaffected in the mutant, we next investigate whether the defective BLEC development was caused by defects in cell migration or proliferation. The time-lapse imaging was performed to illustrate the process of loop-like structure formation using the Tg(lyve1b: DsRed; kdrl: DenNTR) transgenic line. In the wild-type, the BLEC progenitors sprouted from the CVP in the lower middle layer of the brain, then migrated along the MsV, which is located in the top layer of the brain, to form the loop-like structures (Fig. 5a, b and Supplementary Movie. 1). By contrast, in the egfl7 mutant, most of the sprouting cells stopped around CVP and failed to migrate, and formation of bilateral loop-like structures that covered the optic tectum was blocked (Fig. 5a, c and Supplementary Movie. 1). Furthermore, we analyze the expression of proliferating cell nuclear antigen (PCNA) in BLECs that shows individual cells outside of the G0-phase of the cell cycle. At 3 dpf, the lyve1b + BLECs exhibited high proliferation in the control larvae, but rarely proliferated in the mutant (Supplementary Fig. 5a–d). Injection of EdU (5-ethynyl-2’-deoxyuridine) confirmed that BLEC proliferation became significantly reduced in the mutant (Fig. 5d–f and Supplementary Fig. 5e–j). The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays showed that TUNEL and lyve1b double-positive cells was hardly detectable in the egfl7 mutant (Supplementary Fig. 6). All these data demonstrate that the egfl7 mutation blocks proliferation and migration of BLECs, but does not induce their apoptosis.
a Schematic diagram showing the lower middle layer and top layer of the vessels in the brain, respectively. Black frames indicate the image area of corresponding panels. CVP (choroidal vascular plexus) is observed in the lower middle layer; MsV (mesencephalic vein) is in the top layer. b Time-lapse image of Tg(lyve1b: DsRed; kdrl: DenNTR) from 66 hpf to 122 hpf show the BLECs (arrows) sprout from CVP (arrowhead at 66 hpf) and migrate along the MsV (arrowhead) to form the lymphatic loop in WT at 122 hpf. n = 15 embryos. c The BLECs (arrows) of egfl7 mutant shows a delayed sprouting from CVP (arrowhead at 66 hpf), fail to migrate along the MsV (arrowhead), and is unable to form the loop at 122 hpf. n = 15 embryos. d–f EdU staining shows the proliferation of BLECs in WT and egfl7 mutant at 6 dpf, the EdU is injected at 56 hpf. Note the EdU+ BLECs is significantly decreased in the mutant (d, e). Arrowheads indicate the EdU+ BLECs in WT. The statistics show the number of EdU+ BLECs in double loops at 6 dpf in WT and egfl7 mutant (f, n = 18 embryos; two-tailed unpaired t-test). Data are represented as mean ± SD. Scale bar, 50 μm.
Overexpression of Vegfc hardly rescues BLEC formation in the egfl7 mutant
BLEC sprouting depends on Vegfc, Vegfd, Ccbe1, and Vegfr3 signaling15. The BLECs populating in the loop-like structures were significantly reduced in the vegfc-/- and vegfd-/- single mutants and completely absent in vegfc-/-vegfd-/- double mutant16. To test whether the roles of Egfl7 in BLEC proliferation and migration were dependent on classic Vegfc/Vegfr3, we injected hsp70l: Vegfc-P2A-Venus plasmid to generate an ectopic, mosaic expression of Vegfc in embryos, using hsp70l: Venus plasmid as control (Fig. 6a). We firstly analyzed trunk lymphangiogenesis to ensure the Vegfc overexpression. The trunk LECs firstly sprout from the PCV dependent on Vegfc/vegfr3 signaling, and they are highly proliferative as well as migratory when colonizing the HM to form PLs11,38,39,40. After heat-shock, the ectopic expression of Vegfc, but not the control Venus protein, resulted in the hyper-proliferation and hyperbranching of venous-derived ECs prominently in the HM, either in WT or mutant (Fig. 6a, b, d, f, g and Supplementary Fig. 7a, b, d, f). These results ensure that the Vegfc overexpression experiment is working. Next, we overexpress Vegfc in the brain. In contrast to the Venus protein, the ectopic expression of Vegfc was ineffective to the BLEC deficiency in the egfl7 mutant (Fig. 6c, e, h and Supplementary Fig. 7c, e, g). These observations suggest that the roles of Egfl7 in BLEC development doesn’t seem to act upstream of Vegfc.
a Schematic diagram showing the experimental design for detecting the development of LECs after the ectopic, mosaic expression of Vegfc and Venus. Illustrations of the method, transgenic lines, plasmids, and time points of heat shock, and imaging. b, d Compared to the heatshock of Venus, overexpression of Vegfc, causing a hyperbranching in adjacent PLs (arrows) and hyperbranched ISVs in the horizontal myoseptum (arrowheads) of WT (b) and egfl7 mutant (d). f, g The statistics shows the number of PLs per 6 somites (f) and the number of hyperbranched ISVs (g) after ectopic expression of Vegfc and Venus in the trunk (n = 10 embryos; two-tailed unpaired t-test). c, e Compared to the heatshock of Venus, overexpression of Vegfc in the brain does not rescue the devoid of BLECs in the egfl7 mutant. h Quantification of the number of BLECs in the brain after ectopic expression of Vegfc and Venus in the brain (n = 10 embryos; two-tailed unpaired t-test). Data are represented as mean ± SD. Scale bar, 50 μm.
Egfl7 promotes BLEC formation through integrin αvβ3 under the regulation of ILK
Egfl7 is deposited in the ECM upon secretion from endothelial cells, and it has been reported as an integrin ligand strongly associated with fibronectin22. To understand potential mechanisms involved in the egfl7-regulated BLEC formation, integrins previously reported to be expressed by LECs to regulate lymphatic development were analyzed. Among at least 24 unique integrins subunits, integrin α5β1 attached to the ECM to trigger VEGFR3 phosphorylation and LEC proliferation41; integrin α4β1 plays a direct role in regulating lymphangiogenesis42. So, we first carried out FISH and antibody staining and showed the expression of integrins including itga4, itga5, itgb1a, and itgb1b in BLECs (Supplementary Fig. 8a–f). To investigate the roles of integrin α5β1 in BLEC development, we applied an integrin α5β1 inhibitor43, ATN-161, to treat the larvae from 54 hpf to 5 dpf. The BLEC formation remained normal after ATN-161 treatment, with no notable differences in contrast to the control group (Supplementary Fig. 8g, h, k). We then examined BLEC formation using a previously reported itga4cas010 mutant44. Additionally, when IgG647 was injected into the ventricle at 5 dpf, normal BLEC uptake was observed in the itga4cas010 mutant (Supplementary Fig. 8i–k). Thus, these findings suggest that integrin α5β1 and α4β1 are not involved in the Egfl7-driven BLEC formation.
Recent studies have shown that out of all the integrins, αvβ3 is a specific receptor of EGFL724. The expression of itgb3b, itgb3a, and itgav was detected in the sprouting BLECs and CVPs from 54 hpf to 4 dpf (Fig. 7a–h). Additionally, we performed co-immunoprecipitations in HEK293 T cells to confirm the physical association of Egfl7 with Itgav and Itgb3b (Fig. 7i). To examine the impact of integrin αvβ3 on BLEC formation, we treated the Tg(lyve1b: DsRed; kdrl: DenNTR) transgenic line with an inhibitor of Integrin αvβ3, Cilengitide45,46, from 54 hpf to 5 dpf. This inhibitor blocked the formation of BLEC bilateral loop-like structures (Fig. 7j–l). Additionally, we used an alternative knockdown approach and took advantage of the CRISPRi technology (dCas9-KRAB) to inhibit itgb3b transcript elongation47. Compared with the uninjected group (Control), the relative itgb3b expression levels quantified by qPCR on pools of embryos were decreased in the dCas9-KRAB mRNA and itgb3b gRNA co-injected group (Fig. 7o). Moreover, injected dCas9-KRAB mRNA and itgb3b gRNAs could inhibit BLECs loop-like structure formation in the brain (Fig. 7m–p). These results indicate that Egfl7 interacts with integrin αvβ3 to regulate the BLEC development.
a, b, g Schematic diagram showing the lateral view, the lower middle layer, and the top layer of the vessels in the dorsal view, respectively. Black frames indicate the orientation and the image area of corresponding panels. c–f In Tg(lyve1b: GFP; kdrl: DenNTR) transgenic background, FISH and antibody staining show itgb3a and itgb3b are expressed in CVPs at 54 hpf (c, n = 20/21 embryos, e n = 20/20 embryos, arrowheads), and expressed in BVs and departed BLECs at 3 dpf (d, n = 20/20 embryos, f, n = 19/20 embryos). h Double labeling of FISH-itgav and anti-GFP in Tg(lyve1b: GFP) transgenic background at 4 dpf, arrowheads points the itgav expressed in BLECs. n = 15/16 embryos. i HEK293T cells were transfected with Egfl7-Myc and Itgb3b-Flag or Itgav-HA. Egfl7 was immunoprecipitated using anti-Myc antibody and the immunoprecipitants were analyzed for the presence of Itgav-HA or Itgb3b-Flag by Western blot. j–l Inhibition of integrin αvβ3 by Cilengitide treatment from 54 hpf to 5 dpf phenocopy the devoid of the lymphatic loop in the brain (k). Arrows point to the BLECs in the control group (j). The statistics show the number of BLECs in the double loops after Cilengitide treatment (l, n = 15 embryos; two-tailed unpaired t-test. Data are represented as mean ± SD). m–p The itgb3b transcript elongation inhibition causes BLECs reduction in the brain. Dorsal view of BLECs loop-like structure of uninjected siblings and samples co-injected with dCas9-KRAB mRNA and itgb3b gRNAs (m, n). The co-injection of gRNA and CRISPRi results in decreased expression of itgb3b and the number of BLECs (o, n = 3 replicants; p, n = 23 embryos; two-tailed unpaired t-test. Data are represented as mean ± SD). Scale bar, 50 μm.
It has been reported that the integrin-linked kinase (ILK) interacts with integrin in quiescent LECs to prevent non-physiological hyper-activation of VEGFR3 signaling. However, when integrin binds to the ECM, mechanical stimulation disrupts the association of ILK and integrin, releasing the integrin to interact with VEGFR3 and induce VEGFR3 tyrosine phosphorylation48. The expression of ilk could be detected in the BLECs at 4 dpf. (Fig. 8a). To explore the roles of ILK in the Egfl7-involved BLEC formation, we incubated the wild-type and egfl7 cq180 with cpd22, an inhibitor of ILK, from 54 hpf to 6 dpf. The cpd22 partially rescued the BLEC development in the egfl7 mutant (Fig. 8b–g). In addition, we generated an ilk mutant, but it caused severe myocardial dysfunction and deformation, as previously described (Supplementary Fig. 9)49. Therefore, we used the ilk heterozygous mutant for the rescue experiment. The results showed that the absence of loop-like structures in the egfl7 mutant was partially rescued when it was crossed with the ilk heterozygous mutant (Fig. 8h–k). These results demonstrate that even without Egfl7, downregulation of ILK could release a portion of integrin αvβ3 to enhance the phosphorylation of Vegfr3, thus promoting lymphangiogenesis (Supplementary Fig. 10).
a FISH and antibody staining shows ilk is expressed in GFP+ BLECs at 4 dpf (arrowheads). n = 17/18 embryos. b–g Inhibition of ILK by cpd22 can partially rescue the absence of BLECs in the egfl7 mutant at 6 dpf (b–e). Quantification of the number of BLECs in the double loops of brain in different treatment groups (f, n = 10 embryos, two-tailed unpaired t test. Data are represented as mean ± SD). The statistics show the percentage of embryos that have double lymphatic loops, single loops, and none of the loops in the brain (g, WT, n = 51 embryos, WT + cpd22, n = 67 embryos, egfl7-/-, n = 116 embryos, egfl7-/- + cpd22, n = 127 embryos, χ2 test).). h–k An egfl7 mutant was crossed with an ilk heterozygote to generate two types of larvae: ilk+/+; egfl7-/- and ilk+/-; egfl7-/-, which were then studied for BLECs loop-structures formation. The larvae were classified into three categories based on their phenotype: Double loops, single loops, and none of the loops (h–j). The results showed that compared to ilk+/+; egfl7-/-, the ilk+/-; egfl7-/- larvae had a decreased percentage of the none of the loops phenotype, and a partially rescued percentage of double loops and single loops phenotype (k, ilk+/+; egfl7-/-, n = 49 embryos, ilk+/-; egfl7-/-, n = 47 embryos, χ2 test). Scale bar, 50 μm.
Discussion
From these findings, we proposed that in the WT, Egfl7 is deposited in the ECM upon secretion from BECs and LECs. Then, Egfl7 activates Integrin αvβ3, which disrupts the Integrin-ILK association and increases the Vegfr3 phosphorylation, in turn promotes BLEC proliferation. In the egfl7 mutant, ILK interacts with Integrin αvβ3, and Vegfr3-Integrin αvβ3 dissociates, thus reducing Vegfr3 phosphorylation and inhibiting BLEC proliferation (Supplementary Fig. 10). These observations reveal that Egfl7-Integrin αvβ3 mediated ILK-Vegfr3 signaling plays a crucial role in regulating the migration and proliferation of BLECs.
It has been noted that Egfl7 plays a role in vascular repair, CNS inflammation, and cancer angiogenesis. We investigated its relevance in ischemic stroke by examining the expression of Egfl7 during brain vascular injury. We found that Egfl7 is activated not only in both ingrown lymphatic vessels and regenerating blood vessels but also highly expressed in all uninjured BLECs and blood vessels, indicating its significance role in BLECs formation and blood vessel regeneration. In egfl7 cq180, we observed a lack of BLECs but normal BV development, and the reduced BLECs resulted in the failure of lymphatics response to blood vascular injury20, leading to brain edema and death. Despite the absence of BLECs, the egfl7 cq180 can mature into adulthood and appear normal and fertile under bright field imaging. However, it exhibits abnormal meningeal blood vasculature16. Further studies are needed to determine if the absence of BLECs affects the behavior and neurons of adult zebrafish.
The egfl7 cq180, is similar with egfl7 s981, does not show any apparent vascular abnormalities, which can be explained by the activation of compensatory genes-Emilin3. It contains the EMI domain, can regulate vascular elastogenesis29. However, the upregulation of Emilin3 cannot replace the EGF domain, and therefore, it may not be able to rescue the egfl7 cq180 on BLECs formation. Further investigation is required to determine the functional role of different Egfl7 protein domains and ascertain whether the lack of BLECs is due to the absence of the EGF domain.
Integrin, as another receptor of lymphatic endothelial cells, has been reported in lymphangiogenesis. Integrin is a transmembrane receptor, which binds to extracellular matrix components, and is essential for “outside-in” and “inside-out” signaling of the cell, thereby transducing mechanical stimulations8. Surprisingly, our research has found that integrin α4β1 and α5β1 are not essential in the Egfl7-driven lymphatic sprouting in the brain. Instead, integrin αvβ3, which specifically binds to Egfl724, plays a crucial role in brain lymphangiogenesis. We have observed that inhibiting integrin αvβ3 with Cilengitide blocks brain lymphangiogenesis in zebrafish, mimicking the phenotype of the egfl7 cq180. Furthermore, pharmacological inhibition of ILK has rescued the absence of BLECs caused by egfl7 mutation. These findings suggest that EGFL7 regulates BLECs formation by binding to integrin αvβ3, while Ilk interacts with integrins to facilitate mechanically regulated VEGFR3 signaling. Our future research will involve generating the itgav, itgb3a, and itgb3b mutant by Cas9 to mimic the Cilengitide inhibition experiment and the conditional knock out of ilk mutant to rescue the egfl7 cq180. These results also suggest that compared with trunk lymphangiogenesis depending on Vegfc-Ccbe1-Vegfr3, brain lymphatics development is primarily depending on Egfl7-Integrin αvβ3 signaling.
The specific functions of Egfl7 in the endothelial system have been a topic of controversy due to varying phenotypes observed in different knockout (KO) alleles of EGFL7 in mice. Some studies have reported angiogenesis deficits in Egfl7 gene-trap and lacZ knock-in mice50, while Egfl7Δ/Δ mice appeared to be normal28. Although the earlier study attributed vascular phenotypes of Egfl7 KO to the loss of miR-126, the Egfl7-/- that maintains miR-126 expression in another study demonstrates Egfl7 is crucial for placental vascularization and embryonic growth51, suggesting a specific role for Egfl7 in vascular development. Zebrafish BLECs correspond to the mouse leptomeningeal LECs (LLECs)17. And Egfl7 was increased in CNS vasculature of mice with experimental autoimmune encephalomyelitis (EAE). Egfl7-KO or EC-restricted Egfl7-KO mice showed earlier onset of EAE33. Except that, the EAE could induce VEGFR3-dependent lymphangiogenesis52. These studies provide indirect evidence that brain lymphangiogenesis of mice also correlated with Egfl7. However, due to the varying phenotypes observed in different Egfl7-/- mice, further investigations are needed to determine the appropriate Egfl7 mutant of mice for researching the molecular mechanisms of Egfl7 on brain lymphangiogenesis.
Our research has significant implications for the development of brain lymphatics. Firstly, we have discovered that Egfl7 is the primary regulator for brain lymphatic sprouting in zebrafish, rather than Vegfc. Secondly, we have found that egfl7 may not be necessary for angiogenesis, but it plays a crucial role in lymphatic development, particularly in brain lymphangiogenesis. Thirdly, our study provides evidence that the development of BLECs is regulated by specific lymphatic growth factors different from classic signaling involved in trunk lymphangiogenesis. Egfl7 is essential for brain lymphatics development, and its implications are relevant for both normal and pathological conditions. Disruption of brain lymphatic function has been linked to neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. Further research on Egfl7 could uncover new therapeutic targets and improve outcomes for patients with neurodegenerative diseases, making it an exciting area of study in the field of lymphatics biology.
Methods
Ethical Approval
The zebrafish facility and study were approved by the Institutional Review Board of Southwest University (Chongqing, China). Zebrafish were maintained in accordance with the Guidelines of Experimental Animal Welfare from Ministry of Science and Technology of People’s Republic of China (2006). The protocols used for animal experimentation were approved by the Institutional Animal Care and Use Committee protocols from Southwest University (IACUC, 2007). The IACUC number is: IACUC-20231201-01.
Zebrafish strains
The Tg(kdrl:DenNTR)cq10 20, Tg(lyve1b:GFP)cq8621, Tg(lyve1b:DsRed)cq2720, Tg(kdrl:CFP-NTR)cq6220, Tg(prox1:KalTA4;UAS:TagRFP)nim5Tg34, Tg(egfl7:YFP) cq181, Tg(kdrl:egfl7-p2A-GFP) cq182, Tg(lyve1b:egfl7-p2A-GFP) cq183, egfl7 cq180, and itga4cas010 44 (a gift from Weijun Pan), ilk cq187 mutant were used in this study. Embryos were treated with 0.003% 1-phenyl-2-thiourea (PTU, Sigma) to inhibit pigment formation. The sex of zebrafish aged from 1 dpf to 12 dpf was unknown. For adult zebrafish imaging, both males and females were used for this experiment. All the strains are used as stable, germline transgenic lines in this study. Administering general anaesthesia to zebrafish using pharmaceutical-grade buffered Tricaine (MS222, Sigma).
Molecular cloning
For egfl7 promoter, from −3.5 kb to −1.5 kb genomic sequence upstream from the transcription start site combined with exon 1 and a 0.94-kb enhancer sequence located on intron 1 of the egfl7 gene was amplified from genomic DNA. The two pair primers: Egfl7 (−3.5_−1.5)-FW: 5′-CTTCCCCCACTGATAACACATAC-3′; egfl7 (−3.5_−1.5)-RE: 5′- AGGCATGCAGACATGCTCAAGA-3′; Egfl7 (EP)-FW: 5′- GAGGGCCGAGGCGGGAGTGTTTATG-3′; egfl7 (EP)-RE: 5′- CGCTATGCTAAAATCCAGTTGGGCA-3′ were used. Then the two fragments were combined by overlap PCR and subcloned into the pBluescript-kdrl vectors between the XhoI and EcoRI sites to replace the kdrl promoter. To construct the egfl7:YFP plasmid, YFP fragment in pGEMT-YFP was cleaved by SpeI and XmaI, and subcloned downstream of egfl7 promoter to generate the pBluescript -egfl7:YFP construct.
For constructing the pBluescript-kdrl-egfl7-p2A-GFP plasmid and pT2KXIGD-lyve1b-egfl7-p2A-GFP plasmid, we first amplified the full-length egfl7 cDNA by the primers egfl7-CDS-FW: 5’-ACCGGTATGTACACAGCGCTTCTGCTC-3’ and egfl7-CDS-RE: 5’-GTTTTCCTGACAGCCACAGGCTC-3’, then the egfl7 sequence overlapped with the p2A-GFP sequence was inserted between the AgeI and NotI sites in the kdrl:Dendra2 plasmid to replace the Dendra2 fragment. This was done to construct the pBluescript-kdrl:egfl7-p2A-GFP. The pT2KXIGD-lyve1b-egfl7-p2A-GFP plasmid was created using the In-Fusion cloning method (Clontech, #639619). Initially, we designed PCR primers to amplify the egfl7-p2A-GFP fragment with an added 5’ tail, creating 15 bp overhangs for annealing to the vector. The In-Fusion primers used were 5’-AATCCAAGGGATCCA-3’ and 5’-TCTGGATCATCATCG-3’. We then linearized the pT2KXIGD-lyve1b: GFP plasmid between the AgeI and ClaI sites to obtain the vector. The pT2KXIGD-lyve1b-GFP plasmid was kindly provided by Philip S. Crosier. Finally, we performed the In-Fusion reaction to ensure the egfl7-p2A-GFP fragment was inserted into the vectors and validated the fusion points for any mutations via sequencing.
For constructing pcDNA3.1-egfl7WT-Myc and pcDNA3.1-egfl7mut-Myc, pcDNA3.1-Itgb3a-Flag, and pcDNA3.1-itgav-HA plasmids for cell transfection. cDNA was generated using Omniscript reverse transcriptase (Qiagen, #205113). cDNAs encoding the Egfl7WT (831 bp/277 aa), Egfl7mut (213 bp/71 aa) proteins were PCR-amplified using cDNA from WT or egfl7-/- as template, followed by ligated into the mammalian expression vector pcDNA3.1 Myc-tag by In-fusion cloning, the Myc-tag was fused to the C-terminal of the cDNAs. CDS encoding full length of Itgav and itgb3a protein were PCR-amplified. These PCR fragments were ligated into the mammalian expression vector pcDNA3.1 HA-tag or pcDNA3.1 Flag-tag by In-fusion method. All constructs were verified by sequencing.
Generation of transgenic line
For generation of Tg(egfl7:YFP) cq181 transgenic line. Co-injection of I-SceI (NEB) with pBluescript -egfl7:YFP construct flanked by the I-SceI restriction sites into AB genetic background at the one-cell stage for transgenesis. The embryos were screened for transient expression of YFP in endothelium after injection for 3 days by using the Leica epifluorescence microscope. The vasculature of injected embryos showing specific expression of YFP was raised to adulthood and crossed with AB fish to get the germline transmissions. In the stable transgenic line, all the endothelial cells including lymphatic endothelial cells and blood vascular endothelium express the fluorescent protein YFP.
To rescue Egfl7 in BVs and LECs of egfl7 cq180, two stable transgenic lines were generated - Tg(kdrl:egfl7-p2A-GFP) cq182 and Tg(lyve1b:egfl7-p2A-GFP) cq183, respectively. The Tg(kdrl:egfl7-p2A-GFP) was created using the pBluescript vector. The constructs flanked by the I-SceI restriction sites were co-injected along with I-SceI (NEB) into the egfl7 mutant at the one-cell stage for transgenesis. After injection, the embryos were screened for transient expression of GFP under a Leica epifluorescence microscope. The embryos that exhibited specific expression of GFP in the vasculature were raised to adulthood for identification of founder fish with germline integration. To generate Tg(lyve1b:egfl7-p2A-GFP), the plasmid pT2KXIGD-lyve1b-egfl7-p2A-GFP (25 ng/μl) with transposase mRNA (25 ng/μl) were co-injected into one-cell stage of egfl7 mutant embryos and the progeny screened for germline transmission. In the stable transgenic line, all lymphatic endothelial cells express the fluorescent protein-GFP.
Generation of genetic mutants and genotyping
The CRISPR-Cas9 technique was used to generate the mutants. The gRNA target sequence of egfl7 and ilk showed in the Figures, and the gRNAs were synthesized as described53,54. Co-injection of Cas9 protein and gRNAs into one-cell stage of AB genetic background, the genomic region flanking gRNA target site was amplified with the pairs of gene-specific primers including: egfl7 ID-F: 5′-GGAAGTCAACAGCACCTTGAGGG-3′, egfl7-ID-R: 5′-CCTGCCTATAAGAAACCTTGTAG-3′; ilk-ID-F: 5′-GAACAAGATCAACGAGAACC-3′; ilk-ID-R: 5′-TATCGCAATAACTAGCAGCC-3′; itga4-ID-F: 5′-GGTGCTCTGACTGATGACGA-3′, itga4-ID-R: 5′-ATAGGTACAATCCGCGCAAC-3′44, and used these primers to do the PCR and sequenced for validation. The validated embryos were raised to adults (F0). The F0 fishes were screened to identify the founders whose progenies carried indels in these genes. The F1 embryos from the identified F0 were raised to adult, and every F1 adult was identified by genotyping and sequence.
CRISPR interference
The pXT7-dCas9-KRAB plasmid47 was linearized by BamH1 restriction enzymes to serve as templates to synthesize stable mRNAs. Synthesis and purification of dCas9-KRAB mRNA were described previously. The two itgb3b gRNAs were designed to target the non-template strand of the 5′ untranslated region (UTR) and exon 1 of itgb3b to block transcriptional elongation. The target sites are 5′-GCTGTTCTTCTCTACTTCAC-PAM-3′ and 5′-GTAAACCCATAAGCTGAAGAGTT-PAM-3′, and the gRNAs were synthesized as described above. mRNA of dCas9-KRAB (400 ng/μl) and two itgb3b-gRNAs (200 ng/μl) were co-injected into 1-cell stage zebrafish embryos.
Real-time quantitative PCR
Total RNA of egfl7 cq180, wildtype, and embryos that injected with dCas9-KRAB mRNA and itgb3b gRNAs were prepared using the TRIzol reagent (Invitrogen, #15596026). cDNA was generated using Omniscript reverse transcriptase (Qiagen, #205113) with specific stem–loop primers for miRNA. Real-time quantitative PCR was performed using SYBR Green (Roche, #04913914001). The relative miRNA amount was calculated with the ΔΔCt method and normalized with internal control U6 snRNA as previously described55. The egfl7 mRNA expression was normalized by transcriptions of β-actin. RT primer for miR-126: 5′-TGGAGCGACCGTGTCGTGGAGTCGGCTAATGGTCGCTCCATGCAC-3′;Primers for miR-126a/b Real-Time PCR: 5′-GACACTCCAGCAGCGTCGTACCGTGAGTAATA-3′ and 5′-ATAGAGCGGTGTCGTGGAGTCGGCTAATGGTC-3′; Primers for u6 Real-Time PCR: 5′-ACTAAAATTGGAACGATACAGAGA-3′ and 5′-AAAGATGGAACGCTTCACG-3′. Primers for egfl7 qPCR ex1: 5′- ATGTGACCTGCACACGTCAG-3′ and 5′- CACACGACGACTCCAGACAT-3′; Primers for egfl7 qPCR ex2: 5′-ATGTGCCAAAACCACCACATG-3′ and 5′-GAGCCTCCGTTTGCACAAGACT-3′; Primers for itgb3b qPCR ex2: 5′-GATGTTGGACTAGGTTCTAACGT-3′ and 5′-CTGAGGCCTTGTCACTGAGATC-3′; β-actin-qPCR primers: 5′-CGTCTGGATCTAGCTGGTCGTGA-3′ and 5′-CAATTTCTCTTTCGGCTGTGGTG-3′.
Cell transfection and Western blot
HEK293T cells were purchased form Cell Bank, Chinese Academy of Sciences (Shanghai, China), the cells were maintained in DMEM medium (Gibco, #11965092) (containing 10% FBS (Gibco) and 1% penicillin & streptomycin) and cultured at 37 °C, 5% (v/v) CO2. Transfection was performed with Lipofectamine™ 3000 Transfection Reagent (Invitrogen, #L3000-001) and tested according to the protocol provided by the manufacturer.
Cells were lysed with lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1.0% Triton X-100, pH 8.0) containing PMSF (1 mM). Western Blot followed the standard protocol. Briefly, the protein was separated on 12% polyacrylamide gels (Bio-Rad) and transferred in a PVDF membrane. The membranes were blocked with 5% milk and incubated with the following primary antibodies: Mouse Anti-Flag (1:5000, Sigma, #F1804), Mouse Anti-HA (1:5000, Covance, #E11FF01244), Rabbit Anti-Myc (1:5000, Abcam, #ab9106). After 4 °C overnight incubating with primary antibodies, the membrane was washed 5 times with blotting buffer and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies: Goat anti-mouse IgG HRP (1:5000, CST, #91196), Goat Anti-Rabbit IgG HRP (1:5000, CST, #7074) for 2 h at room temperature. After washing the membrane, SuperSignal West Pico Chemiluminescent Substrate (#34577, Thermo Fisher Scientific,) was used to visualize by chemiluminescence.
Co-immunoprecipitation
For Egfl7-Itgb3b and Egfl7-Itgav coimmunoprecipitations, 5 μg pcDNA3.1-Egfl7-myc, pcDNA3.1-Itgb3b-Flag, pcDNA3.1-itgav-HA plasmids and different combinations as indicate on demand were transfected into HEK293T cells. The cells were collected after 36 h of incubation and the total cell lysates were prepared using lysis buffer. Take 1/3 of total cell lysates as input and detected the protein level by Western blot. The remaining lysates were subjected to immunoprecipitation with Rabbit anti-Myc (1:5000, Abcam, #ab9106) using Pure Proteome™ Protein A/G Mix Magnetic Beads (Millipore, #LSKMAGAG02) according to the manufacturer’s instructions. Coimmunoprecipitated proteins were eluted in 2× SDS sample buffer (125 mM Tris, pH6.8, 20% glycerol, 0.02% bromophenol blue, 2% b-mercaptoethanol, and 4% SDS), separated on 12% SDS-PAGE gels, and analyzed by immunoblotting using anti-HA (Covance), anti-Myc (Abcam), or anti-Flag antibodies (Sigma).
Whole-mount in situ hybridizations and immunofluorescence staining
The zebrafish embryos at indicated stages were fixed in 4% PFA for in situ hybridizations56. To avoid of off-target hybridization to tissues expressing transgenes, the digoxigenin-labeled antisense RNA probe egfl7 was synthesized from PCR templates. Then the whole-mount in situ hybridizations was carried out as previously described. Images were snapped using a SteREO Discovery V20 (Carl Zeiss) microscope equipped Zen 2011 software.
The embryos for whole mount immunofluorescence staining were fixed in PEM and 2% FA at 4 °C overnight57. Then incubated the following primary antibodies including: Anti-PCNA (1:500, Sigma, #SAB2701819), Anti-DsRed (1:1000, Santa Cruz, #sc-101526), Anti-Dendra2 (1:1000, Antibody-online, #ABIN361314), Anti-Prox1 (1:500, Abcam, #ab5475). After washing with PBST several times, incubated the embryos with Alexa fluorescent-conjugated secondary antibodies (1:1000, Invitrogen) at 4 °C overnight and washed to subjected for mounting and imaging under Confocal microscope (LSM 880, Carl Zeiss). Antibody stained embryos were mounted in 1.2% low melting point agarose and imaged using ZEN2010 software equipped on an LSM 880 confocal microscope (Carl Zeiss).
Combined FISH and antibody staining
PCR-amplified sequences of egfl7, vegfr3, mrc1a, itga4, itga5, itgb1b, itgb3a, itgb3b, itgav, and ilk were used as templates for the synthesis of antisense digoxigenin-labeled RNA probes21.
The combination of FISH and antibody staining was performed as previously described. In short, after being fixed in 4% PFA at 4 °C overnight, the larvae older than 4 dpf were manually removed the skin under the microscope as previously described58,59. Then, the larvae were dehydrated in methanol at −20 °C for at least 24 h. The dehydrated samples were serially transferred into methanol in PBST (1% Triton X-100 in PBS) and pre-hybridized in the HYB buffer (50% formamide, 5×SSC, 0.1% Tween-20, 5 mg/ml torula yeast RNA, 50 mg/ml heparin) and hybridized with the digoxigenin-labeled egfl7, vegfr3, mrc1a, itga4, itga5, itgb1b, itgb3a, itgb3b, itgav, and ilk probes at 65 °C overnight. After removal of probes, the larvae were serially washed with SSCT and MABT (150 mM maleic acid, 100 mM NaCl, 0.1% Tween-20, pH 7.5), then blocked in 2% Block Reagent (Roche, #11096176001) and incubated with the Anti-digoxigenin POD antibodies (1:500, Roche) overnight. The larvae were serially washed MABT, PBST, and PBS, then incubated in TSA Plus Fluor or Cy5 Solution (Perkin Elmer, #NEL745) overnight and washed again with PBST. Afterwards, these samples were subjected to do the antibody staining.
Tg(lyve1b:GFP; kdrl:DenNTR) lines were subjected to antibody staining using the anti-GFP (1:1000, Santa Cruz, #sc9996) and anti-Dendra2 (1:1000, Antibody-online, #ABIN361314) primary antibodies. Then, the goat anti-mouse IgG Alexa fluor 633-conjugated (1:1000, Invitrogen, #A21052) and goat anti-rabbit IgG Alexa fluor 405-conjugated (1:1000, Invitrogen, #A31556) secondary antibodies were used to label GFP and Dendra2, respectively.
TUNEL assay and ICV injection
Larvae were fixed in 4% PFA at 4 °C overnight, subjected to skin removal, and assayed using the In-Situ Cell Death Detection Kit, TMR Red (Roche, #04913914001) according to the manufacturer’s instruction. And the positive control was generated by treating samples with DNAseI to cause DNA breakage before staining.
After embryos were mounted in 1.2% low melting agarose, a suspension of IgG-conjugated Alexa Fluor 647 (2 mg/ml, Invitrogen) was injected into the center of the optic tectum using glass capillary needles.
EdU staining
The larvae were fixed in 4% PFA overnight at 4 °C, followed by removal of the skin. The Click-iT™ EdU Alexa Fluor™ 647 Imaging Kit (Invitrogen, #C10340) was used to assay the samples. EdU (500 μM) was injected into the CCV and ventricle of the brain in both WT and egfl7 mutant at 56 hpf or 3 dpf, as mentioned in the experiment. The samples were then fixed at the specified timepoints. The proliferation cells were labeled with EdU, and the DsRed+ BLECs in the Tg(lyve1b: DsRed) transgenic line were stained with antibodies.
Imaging
For time-lapse live imaging, the embryos were mounted in 1–1.2% low melting point agarose in the egg water with 0.003% PTU using 35 mm glass bottom dishes, and the Z-stack images of about 24 embryos were snapped every 2 h by LSM880-confocal microscope equipped with ZEN2010 software (Carl Zeiss).
For the dissected adult brain imaging, the adult was firstly anesthetized with 40 mg/L Tricaine (MS222, Sigma), then the brains were dissected and placed into cold PBS for imaging. The dissected brains were whole-mount imaged by LSM880-confocal microscope equipped with 10X Air (0.45 N.A.) and 20X water immersion (0.95 N.A.) objectives using ZEN 2010 software. The large size of the adult zebrafish brains required tile scan acquisitions that were later stitched using ZEN 2010 software.
For adult fish live imaging, the WT and egfl7 mutant under Tg(lyve1b: DsRed) transgenic line (older than 6 months post fertilization, males or females) were anesthetized with 140 mg/L tricaine (MS-222) and placed upright or sidewise into a sponge slit moistened with tricaine water. The sponge containing the fish was placed in a petri dish filled with tricaine water. The fluorescence of lateral head and the dorsal head were snapped by Leica M205 FCA stereomicroscope equipped with LAS X software.
Chemical treatment
For NTR-Mtz ablation experiment, the larvae at 3 dpf were incubated with 2 mM Metronidazole (Sigma, #M3671) dissolved in 0.2% DMSO for 2.5 h, then washed the larvae with fish water three times and recovered in embryo medium containing 0.003% PTU. After about one day, the brain vascularity of these Mtz-treated larvae showed ablation by Mtz and can be examined under the fluorescent microscope. The integrin α5β1 inhibitor-ATN-161(Selleck, #262438-43-7) and the integrin αvβ3 inhibitor-Cilengitide (MCE, #HY16141) stock solution of 10 mM in DMSO was used to prepare a working solution-50 μM and treat the embryos from 54 hpf to 5 dpf. 2.5 μM cpd22 (Calbiochem, 407331), an ilk inhibitor, dissolved in DMSO and diluted in fish water was used to treat the embryos from 54 hpf to 6 dpf.
Mosaic ectopic expressions
For ectopic expression of Vegfc and Venus, the plasmid pBluscript-hsp70l-Vegfc-p2A-Venus-cryaa-Venus20 and pBluescript-hsp70l-Venus19 were used to inject into the blastomeres of egfl7 mutants in Tg(lyve1b: DsRed) transgenic line at the one-cell stage20. Then, heat-shock was performed at the indicated time points. Heat shock was carried out at 38.5 °C for 40 min in the water bath. After heat-shock for several hours, the larvae showed green fluorescence under a microscope.
Quantification and statistical analysis
All statistical calculations were performed using GraphPad Prism. Variance for all groups data is presented as ± SD, or Boxplot with Min to Max whiskers. All experiments were performed with embryos and adult zebrafish. The embryos were collected from in-crosses and outcrosses of several pairs of adult zebrafish. egfl7 mutant was grown in a single tank, the other mutants and sibling larvae were grown together in a single tank. Phenotyping preceded genotyping in mutant analyses, hence analysis was genotype blinded. In the other experiments, the investigators were not blinded to group allocation during data collection and/or analysis. All experiments comparing treatment groups were carried out using randomly assigned siblings. After at least two repeated experiments (n numbers is indicated in the figure legends), data were analyzed for statistical significance using Two-way ANOVA by Sidak’s multiple comparisons test, Chi-square test, and two-tailed unpaired t-test. A value of p < 0.05 was considered to be statistically significant. No data were excluded from analyses. The exact sample size (n), p-value for each experimental group, statistical tests, and error bars (e.g. SD, SEM) were defined in the figure legends or in the Source Data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated and/or analysed in this study are available in the Article and the Supplementary Information. If there is potential for commercial application, we may require a payment or a completed Material Transfer Agreement. All zebrafish lines and plasmids generated in this study are available from the laboratory of L.L. (lluo@fudan.edu.cn). Source data are provided with this paper.
References
Oliver, G., Kipnis, J., Randolph, G. J. & Harvey, N. L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 182, 270–296 (2020).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 21, 1505–1513 (2002).
Johnson, N. C. et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 22, 3282–3291 (2008).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Hogan, B. M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet 41, 396–398 (2009).
Le Guen, L. et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141, 1239–1249 (2014).
Jeltsch, M. et al. CCBE1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971 (2014).
Planas-Paz, L. et al. Mechanoinduction of lymph vessel expansion. Embo J. 31, 788–804 (2012).
Galvagni, F. et al. Endothelial Cell Adhesion to the Extracellular Matrix Induces c-Src-Dependent VEGFR-3 Phosphorylation Without the Activation of the Receptor Intrinsic Kinase Activity. Circulation Res. 106, 1839–U1125 (2010).
Kuchler, A. M. et al. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr. Biol. 16, 1244–1248 (2006).
Yaniv, K. et al. Live imaging of lymphatic development in the zebrafish. Nat. Med 12, 711–716 (2006).
Koltowska, K., Betterman, K. L., Harvey, N. L. & Hogan, B. M. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development 140, 1857–1870 (2013).
Eng, T. C. et al. Zebrafish facial lymphatics develop through sequential addition of venous and non-venous progenitors. EMBO Rep. 20, e47079 (2019).
Okuda, K. S. et al. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391 (2012).
van Lessen, M. et al. Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. Elife 6, e25932 (2017).
Bower, N. I. et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat. Neurosci. 20, 774–783 (2017).
Shibata-Germanos, S. et al. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathol. 139, 383–401 (2020).
Castranova, D. et al. Live Imaging of Intracranial Lymphatics in the Zebrafish. Circ. Res 128, 42–58 (2021).
Chen, J., He, J. & Luo, L. Brain vascular damage-induced lymphatic ingrowth is directed by Cxcl12b/Cxcr4a. Development 149, dev200729 (2022).
Chen, J. et al. Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev. Cell 49, 697–710 e695 (2019).
Chen, J. et al. Acute brain vascular regeneration occurs via lymphatic transdifferentiation. Dev. Cell 56, 3115–313127 e3116 (2021).
Nichol, D. & Stuhlmann, H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood 119, 1345–1352 (2012).
Fitch, M. J., Campagnolo, L., Kuhnert, F. & Stuhlmann, H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev. Dyn. 230, 316–324 (2004).
Nikolic, I. et al. EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood 121, 3041–3050 (2013).
Parker, L. H. et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 428, 754–758 (2004).
Charpentier, M. S. et al. CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway. Dev. Cell 25, 132–143 (2013).
Nichol, D. et al. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood 116, 6133–6143 (2010).
Kuhnert, F. et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development 135, 3989–3993 (2008).
Rossi, A. et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233 (2015).
Gustavsson, M. et al. Vascular response to hypoxic preconditioning in the immature brain. J. Cereb. Blood Flow. Metab. 27, 928–938 (2007).
Wu, F. et al. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology 50, 1839–1850 (2009).
Hong, G. et al. EGFL7: Master regulator of cancer pathogenesis, angiogenesis and an emerging mediator of bone homeostasis. J. Cell Physiol. 233, 8526–8537 (2018).
Larochelle, C. et al. EGFL7 reduces CNS inflammation in mouse. Nat. Commun. 9, 819 (2018).
van Impel, A. et al. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141, 1228–1238 (2014).
Fish, J. E. et al. MiR-126 regulates angiogenic signaling and vascular integrity. Dev.Cell 15, 272–284 (2008).
Kontarakis, Z., Rossi, A., Ramas, S., Dellinger, M. T. & Stainier, D. Y. R. Mir-126 is a conserved modulator of lymphatic development. Dev. Biol. 437, 120–130 (2018).
Venero Galanternik, M. et al. A novel perivascular cell population in the zebrafish brain. Elife 6, e24369 (2017).
Bussmann, J. et al. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137, 2653–2657 (2010).
Cha, Y. R. et al. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev. Cell 22, 824–836 (2012).
Koltowska, K. et al. Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Rep. 13, 1828–1841 (2015).
Zhang, X., Groopman, J. E. & Wang, J. F. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J. Cell Physiol. 202, 205–214 (2005).
Garmy-Susini, B. et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 70, 3042–3051 (2010).
Edwards, D. N. et al. Integrin α5β1 inhibition by ATN-161 reduces neuroinflammation and is neuroprotective in ischemic stroke. J. Cereb. Blood Flow. Metab. 40, 1695–1708 (2020).
Li, D. et al. VCAM-1(+) macrophages guide the homing of HSPCs to a vascular niche. Nature 564, 119–124 (2018).
Li, Y. et al. Antagonizing αvβ3 Integrin Improves Ischemia-Mediated Vascular Normalization and Blood Perfusion by Altering Macrophages. Front Pharm. 12, 585778 (2021).
Pan, X. et al. Cilengitide, an αvβ3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model. Bioengineered 13, 4557–4572 (2022).
Long, L. et al. Regulation of transcriptionally active genes via the catalytically inactive Cas9 in C. elegans and D. rerio. Cell Res. 25, 638–641 (2015).
Urner, S. et al. Identification of ILK as a critical regulator of VEGFR3 signalling and lymphatic vascular growth. Embo J. 38, e99322 (2019).
Knoll, R. et al. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 116, 515–525 (2007).
Schmidt, M. et al. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 134, 2913–2923 (2007).
Lacko, L. A. et al. Altered feto-placental vascularization, feto-placental malperfusion and fetal growth restriction in mice with Egfl7 loss of function. Development 144, 2469–2479 (2017).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Cai, P. et al. Farnesoid X Receptor Is Required for the Redifferentiation of Bipotential Progenitor Cells During Biliary-Mediated Zebrafish Liver Regeneration. Hepatology 74, 3345–3361 (2021).
Chang, N. et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 23, 465–472 (2013).
Zou, J. et al. Two functional microRNA-126s repress a novel target gene p21-activated kinase 1 to regulate vascular integrity in zebrafish. Circ. Res. 108, 201–209 (2011).
Lu, H., Ma, J., Yang, Y., Shi, W. & Luo, L. EpCAM is an endoderm-specific Wnt derepressor that licenses hepatic development. Dev. Cell 24, 543–553 (2013).
He, J. et al. Mammalian Target of Rapamycin Complex 1 Signaling Is Required for the Dedifferentiation From Biliary Cell to Bipotential Progenitor Cell in Zebrafish Liver Regeneration. Hepatology 70, 2092–2106 (2019).
He, J., Lu, H., Zou, Q. & Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800.e788 (2014).
He, J., Mo, D., Chen, J. & Luo, L. Combined whole-mount fluorescence in situ hybridization and antibody staining in zebrafish embryos and larvae. Nat. Protoc. 15, 3361–3379 (2020).
Acknowledgements
We thank Prof. Weijun Pan for the itga4cas010 mutant fish lines. This work was supported by the National Key R&D Program of China (2021YFA0805000 (L.L.)), and the National Natural Science Foundation of China (32322028 (J.C.), 32192400 (L.L.)).
Author information
Authors and Affiliations
Contributions
J.Chen and L.Luo designed the experimental strategy, analyzed data, and wrote the manuscript. J.D. generated the egfl7 mutant and performed the combination of FISH and antibody staining. Y.X and F.F generated the ilk mutant, performed CRISPRi, constructed plasmids, and performed injections. Y.Li and T.W did the chemical treatment, co-immunoprecipitation and western blot experiments. J.H, J.Cang, and L.L analyzed data and provided a discussion. J.Chen performed all the other experiments in the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Ding, J., Li, Y. et al. Epidermal growth factor-like domain 7 drives brain lymphatic endothelial cell development through integrin αvβ3. Nat Commun 15, 5986 (2024). https://doi.org/10.1038/s41467-024-50389-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50389-8