Abstract
Fischer-Tropsch synthesis (FTS) is a structure-sensitive reaction of which performance is strongly related to the active phase, particle size, and exposed facets. Compared with the full-pledged investigation on the active phase and particle size, the facet effect has been limited to theoretical studies or single-crystal surfaces, lacking experimental reports of practical catalysts, especially for Fe-based catalysts. Herein, we demonstrate the facet sensitivity of iron carbides in FTS. As the prerequisite, {202} and {112} facets of χ-Fe5C2 are fabricated as the outer shell through the conformal reconstruction of Fe3O4 nanocubes and octahedra, as the inner cores, respectively. During FTS, the activity and stability are highly sensitive to the exposed facet of iron carbides, whereas the facet sensitivity is not prominent for the chain growth. According to mechanistic studies, {202} χ-Fe5C2 surfaces follow hydrogen-assisted CO dissociation which lowers the activation energy compared with the direct CO dissociation over {112} surfaces, affording the high FTS activity.
Similar content being viewed by others
Introduction
Fischer-Tropsch synthesis (FTS) is a structure-sensitive reaction for the sustainable production of synthetic fuels and building-block chemicals from syngas1,2,3,4,5,6. The catalytic performance is strongly related to the active phase, particle size, and exposed facets of the active components such as iron, cobalt, and ruthenium7,8,9,10,11,12,13. Compared with Co and Ru, Fe-based catalysts have superior properties including resistance to the formation of methane, low cost, high adaptability to broad H2/CO ratios, and versatility to various useful products14,15,16. For Fe-based catalysts, pure-phase iron carbides were synthesized by using Fe(CO)5 reagent, which was explored by means of in-situ characterizations17,18. The Fe3O4@χ-Fe5C2 core-shell catalysts were constructed by pyrolyzing iron-containing metal-organic frameworks, whereas the obtained nanoparticles were irregular spherical particles19. The transformation of reduced iron phases to iron carbides promoted the formation of hydrocarbon species in FTS20. The effects of the active phase and particle size have been extensively studied19,21,22,23,24,25,26. These effects are generally entangled with the contributions of surface terrace, corner, edge, and step-edge sites, where differences in coordination numbers and surface topology may lead to substantial differences in intrinsic performance27. Up to date, the investigation of the facet effect has been limited to theoretical calculations or single-crystal surfaces. For instance, density functional theory (DFT) calculations of CO activation on χ-Fe5C2 surfaces indicated that the terraced (510) surface inclined to directly dissociate CO molecules, whereas the stepped (010) and (001) surfaces preferred the hydrogen-assisted CO dissociation route28. In-situ scanning tunneling microscopy visualized on-surface ethylene polymerization on a carburized Fe(110) single-crystal surface29. However, there is no experimental report on the facet effect of practical Fe-based catalysts due to the complexity and dynamic structural evolution of iron carbides during FTS.
As the widely accepted active phase for FTS, χ-Fe5C2 has a base-centered monoclinic (bcm) structure with space group C2/c (a = 11.56 Å, b = 4.57 Å, c = 5.06 Å, and β = 97.74°)30,31. Owing to the low symmetry of the lattice structure, it remains as a grand challenge to synthesize χ-Fe5C2 nanocrystals with uniformly exposed surfaces. We proposed to use a highly symmetrical template as the core to support the χ-Fe5C2. Fe3O4 has a face-centered cubic (fcc) structure with a high symmetry. Herein, we reported the conformal reconstruction of well-defined Fe3O4 nanocrystals to generate χ-Fe5C2 with specifically exposed surfaces. The samples consisted of an inner core of Fe3O4 and an outer shell of χ-Fe5C2, denoted as Fe3O4@χ-Fe5C2 nanocrystals. We obtained Fe3O4@χ-Fe5C2 nanocrystals with surfaces terminated in {202} and {112} facets of χ-Fe5C2 shells through using cubic and octahedral Fe3O4 as the templates, respectively (Fe3O4@χ-Fe5C2 nanocubes and octahedra, respectively). We discovered that Fe3O4@χ-Fe5C2 nanocubes were more catalytically active and stable than the octahedral counterpart during FTS, whereas the facet sensitivity was not prominent for the chain growth. According to mechanistic studies, the high activity of {202} χ-Fe5C2 surfaces derived from the unique reaction path in which the hydrogen-assisted CO dissociation route lowered the activation energy relative to the direct CO dissociation route over {112} surfaces.
Results and discussion
Synthesis and characterization of Fe3O4@χ-Fe5C2 nanocubes
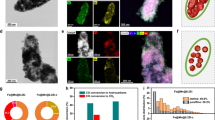
To begin with, Fe3O4 nanocubes were prepared with an average size of 40.5 ± 3.9 nm and a purity of 95.3% (Supplementary Fig. 1). Fe3O4@χ-Fe5C2 core-shell nanocubes were synthesized via surface reconstruction of Fe3O4 under syngas atmosphere. Specifically, Fe3O4 nanocubes were reduced under 1 bar of H2 with a gas-flow rate of 100 mL min−1 at 270 °C for 10 h. This treatment ensured the removal of surface organic species (Supplementary Fig. 2). Afterwards, the obtained samples underwent surface reconstruction in a fixed-bed reactor under 20 bar of syngas (32 vol% H2, 64 vol% CO, and 4 vol% Ar) with a space velocity of 2400 mL h−1 gcat−1 at 270 °C for 20 h. Figure 1a shows the transmission electron microscopy (TEM) image of Fe3O4@χ-Fe5C2 nanocubes. The average size of Fe3O4@χ-Fe5C2 nanocubes was 40.1 ± 3.8 nm with a purity of 90.0% (Supplementary Fig. 3). The Fe3O4@χ-Fe5C2 nanocubes exhibited a surface area of 20.4 m2 g−1 based on the Brunauer-Emmett-Teller (BET) method (Supplementary Fig. 4a). The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image taken from one of the nanocubes revealed a periodic lattice extending across the entire surface (Fig. 1b). The magnified image clearly revealed lattice differences between the edge region and the central region, implying the formation of the core-shell structure (Fig. 1c). In the case of nanocrystals with a core-shell structure, a slight lattice mismatch (f < ~5%) is required for the epitaxial surface layer to form over the inner core32. This epitaxial relationship allows for the maintenance of orientation between the growth layer and the substrate within the first few atomic layers. The spacing of Fe3O4(400) planes (0.21 nm) in the core was approximately equal to that of χ-Fe5C2(202) planes (0.22 nm) in the shell (Fig. 1d, e). The small lattice mismatch (f) of 4.65% calculated from Eq. (1) ensures the preservation of the epitaxial orientation relationship.
a TEM image of Fe3O4@χ-Fe5C2 nanocubes. b HAADF-STEM image of an individual Fe3O4@χ-Fe5C2 nanocube. c Magnified HAADF-STEM image of the region marked by the corresponding box in panel (b). d Intensity profile recorded from the area indicated by the rectangular box in panel (c). e Intensity profile recorded from the area indicated by the rectangular box in panel (c). f FFT pattern from box f in panel (c). g Simulated FFT pattern of Fe3O4 along the [001] direction. h FFT pattern from box h in panel (c). i Simulated FFT pattern of χ-Fe5C2 along the [10-1] direction. j EELS spectra of a Fe3O4@χ-Fe5C2 nanocube in panel (c). k Elemental mapping images of a Fe3O4@χ-Fe5C2 nanocube. l Line-scan profile recorded along the line of the inset HAADF image. m Mössbauer spectra of Fe3O4@χ-Fe5C2 nanocubes.
In Eq. (1), dshell and dcore refer to the lattice spacings of the shell and the core, respectively. The small lattice mismatch results in only one type of facet at the surface layer of Fe3O4@χ-Fe5C2 nanocubes. To identify the corresponding facets, we conducted the fast Fourier transform (FFT) analysis. According to the combination of the experimental and simulated FFT patterns, the region in the inner core was indexed as the (400) facet of fcc Fe3O4 along the [001] zone axis, while that in the outer shell corresponded to the (202) facet of bcm χ-Fe5C2 along the [10−1] zone axis (Fig. 1f–i). The HAADF-STEM images of different corners and edges in an individual nanoparticle indicated that the surfaces exposed a uniform χ-Fe5C2 shell with the {202} facets (Supplementary Fig. 5). The average thickness of the shell was determined as 2.0 nm, approximating ten atomic layers (Supplementary Fig. 5).
The core of iron oxides and the shell of iron carbides were further confirmed through the spatial elemental analysis. Specifically, the electron energy loss spectroscopy (EELS) image implied that the core region mainly comprised Fe and O elements while the shell region contained Fe and C elements (Fig. 1j). The scanning transmission electron microscopy-energy dispersive X-ray (STEM-EDX) analysis including the elemental mapping images and the line-scanning profile confirmed that O and C elements were mainly located in the core and the shell, respectively, while Fe was homogeneously distributed throughout the particle (Fig. 1k, l).
To quantify the contents of different phases in Fe3O4@χ-Fe5C2 nanocubes, we carried out Mössbauer and X-ray diffraction (XRD) characterizations. Based on the Mössbauer results, the sample was composed of 63.4 wt% of Fe3O4, 33.2 wt% of χ-Fe5C2, and 3.4 wt% of Fe(II)/Fe(III) species (Fig. 1m and Supplementary Table 1). Fe(II)/Fe(III) species indicated the presence of some poorly crystallized iron oxides. The quantitative analysis of XRD showed that χ-Fe5C2 occupied 29.8 wt% of the total mass, approaching that (33.2 wt%) obtained from the Mössbauer spectra (Supplementary Fig. 6a and Supplementary Table 2).
Evolution from Fe3O4 nanocubes to Fe3O4@χ-Fe5C2 nanocubes
Figure 2a depicts the schematic of the evolution from Fe3O4 nanocubes to Fe3O4@χ-Fe5C2 nanocubes. The major steps were verified by HAADF-STEM images and XRD patterns. Specifically, after reduction, the initial Fe3O4 nanocubes with {400} facets were transformed into metallic Fe nanocubes with their surfaces terminated in {100} facets (Fig. 2b, c and Supplementary Fig. 6b, c). When metallic Fe nanocubes were exposed to the syngas, the thermodynamic driving force induced the phase transition from metallic Fe to iron carbides. The surface Fe atoms underwent carburization and oxidation by reacting with carbon and oxygen species derived from the dissociated CO. After the exposure to syngas for 2 h, the corners were preferentially carburized and oxidized, resulting in the random distribution of Fe3O4 and χ-Fe5C2 domains (Fig. 2d). Notably, the diffractions of Fe3O4 and metallic Fe were clearly observed, whereas the crystalline χ-Fe5C2 was not detected by XRD (Supplementary Fig. 6d). This result implied that χ-Fe5C2 existed in short-range order with an amorphous structure. When Fe nanocubes were exposed to syngas for 5 h, carbon atoms cleaved from CO completely carburized the metallic Fe at the corner of the nanocube, while the dissociated oxygen atoms permeated into the interior, resulting in the bulk transition into Fe3O4 (Fig. 2e). At this stage, the diffractions of χ-Fe5C2 were observed in the XRD patterns, along with metallic Fe and Fe3O4 (Supplementary Fig. 6e). When Fe nanocubes were exposed to syngas for 10 h, the dissociated carbon atoms carburized the surface layers from the corners to the whole faces (Fig. 2f and Supplementary Fig. 6f). A core-shell structure with the Fe3O4 core and χ-Fe5C2 surface formed as the result of that the nanocubes lost the thermodynamic driving force for further carburization or oxidation33. The core-shell structure did not show obvious change when the syngas treatment was prolonged to 20 h (Fig. 1b, c). When the Fe3O4@χ-Fe5C2 core-shell structure formed at a steady state, the excess dissociated oxygen atoms were released into the gas phase in the form of CO2 and H2O to prevent the oxidation of the shell. The excess dissociated carbon atoms reacted with surface-dissociated hydrogen atoms to yield hydrocarbons rather than to permeate and carburize the Fe3O4 interlayer. The stable core-shell structure was the result of the dynamic balance of the hydrocarbon production, surface oxidation, and carburization in the syngas environment.
a Schematic of the major steps involved in the continuous evolution from the Fe3O4 nanocube to the Fe3O4@χ-Fe5C2 nanocube. I refers to the reduction process; II refers to the corner carburization and oxidation; III refers to the surface carburization, O migration, and bulk oxidation; IV refers to the surface balanced carburization and oxidation. b HAADF-STEM images of an individual Fe3O4 nanocube. c HAADF-STEM images of an individual Fe nanocube. d–f HAADF-STEM images of an individual nanocrystal after the treatment of Fe nanocubes with syngas for 2, 5, and 10 h, respectively.
We conclude the driving force to limit a single type of phase and facet at the surface layer as follows. The driving force to regulate the phase is the carbon chemical potential of reaction conditions. It was reported that the activation energy for carbon diffusion in Fe (43.9–69.0 kJ mol−1) was lower than that for the FTS reaction (89.1 ± 3.8 kJ mol−1)14. Consequently, surface carbon atoms cleaved from CO exhibit a pronounced affinity to Fe atoms, thereby instigating a phase transition from iron to FeCx during the initial stage of the FTS reaction. Upon the formation of active FeCx on the surface, the FTS reaction is facilitated, resulting in the release of oxygen species, predominantly in the form of H2O, into the gas phase. This influx of H2O induces Fe oxide formation and impedes further carburization. As the concentration of oxygen species diminishes in the gas phase, a greater quantity of carbide accumulates on the surface, pushing the reaction condition back to the equilibrium position and vice versa. As the inner core of the catalyst oxidizes to Fe3O4, it becomes more resistant to carbon permeation and carburization compared to metallic Fe. According to carbon chemical potential theory, carbon-rich χ-Fe5C2 is the thermodynamically stable phase under our reaction condition (20 bar, CO:H2 = 1:2, 270 °C)21. As such, other Fe carbides present in the surface layers will evolve into χ-Fe5C2 as the reaction progresses. Moreover, the uniform facet of the substrate and the small lattice mismatch (<~5%) within the core-shell structure ensure the preservation of the epitaxial orientation relationship.
Synthesis and characterization of Fe3O4@χ-Fe5C2 octahedra
For comparison, we applied the surface reconstruction procedure to prepare Fe3O4@χ-Fe5C2 octahedra. We carbonized the synthesized Fe3O4 octahedra following a similar approach to that of nanocubes (Supplementary Fig. 7). The average size of Fe3O4@χ-Fe5C2 octahedra was 45.4 ± 3.5 nm with a purity of 93.3% (Fig. 3a and Supplementary Fig. 8). The surface area of Fe3O4@χ-Fe5C2 octahedra was 22.0 m2 g−1 based on the BET method (Supplementary Fig. 4b). The lattice disparity between the core and the shell was clearly revealed by the HAADF-STEM images (Fig. 3b and Supplementary Fig. 9). The uniform shell had an average thickness of 1.7 nm, corresponding to eight atomic layers (Fig. 3c). We assigned the lattice parameters with the help of the experimental and simulated FFT patterns. Specifically, the inner core took a lattice parameter of 0.48 nm which was indexed as the (111) facet of fcc Fe3O4 along the [001] zone axis (Fig. 3d, f, g). For the outer shell, the lattice parameter of 0.21 nm was assigned to the (112) facet of bcm χ-Fe5C2 along the [10-1] zone axis (Fig. 3e, h, i). Besides lattice matching, the epitaxial orientation can also be preserved via domain matching, where the spacing of m lattice planes in the epilayer is approximately equal to n in the substrate32,34,35. We observed that the spacing of three Fe3O4(111) planes in the core was approximately equal to that of seven χ-Fe5C2(112) planes in the shell (Fig. 3c). Such periodicity leads to a commensurate epitaxial relationship with a low mismatch value of 2.06% according to Eq. (2).
a TEM image of Fe3O4@χ-Fe5C2 octahedra. b HAADF-STEM image of an individual Fe3O4@χ-Fe5C2 octahedron. c Magnified HAADF-STEM image of the region marked by the corresponding box in panel (b). d Intensity profile recorded from the area indicated by the rectangular box in panel (c). e Intensity profile recorded from the area indicated by the rectangular box in panel (c). f FFT pattern from box f in panel (c). g Simulated FFT pattern from of Fe3O4 along the [1−10] direction. h FFT pattern from box h in panel (c). g Simulated FFT pattern from of χ-Fe5C2 along the [20−1] direction. j EELS spectra of a Fe3O4@χ-Fe5C2 octahedron in panel (c). k elemental mapping images of a Fe3O4@χ-Fe5C2 octahedron. l Line-scan profile recorded along the line of the inset HAADF image. m Mössbauer spectra of Fe3O4@χ-Fe5C2 octahedra.
The domain match allows the conformal growth for the (112) facet of χ-Fe5C2 over the (111) facet of Fe3O4. The distribution of Fe3O4 at the core and χ-Fe5C2 at the shell was supported by the EELS image, STEM-EDX elemental mapping images, and line scanning profile (Fig. 3j–l). Mössbauer result indicated that Fe3O4@χ-Fe5C2 octahedra contained 65.1 wt% of Fe3O4 and 29.5 wt% of χ-Fe5C2 (Fig. 3m). The content of χ-Fe5C2 was consistent with the XRD quantitative analysis result (27.6%) (Supplementary Tables 1 and 2).
Facet effect on activity and selectivity
We explored the facet effect of iron carbides on FTS properties. As the shells of Fe3O4@χ-Fe5C2 nanocrystals are more than six atomic layers thick, the electronic coupling between the core and outermost layer in the shell is essentially lost, and thus the ability to access the strain-dependent catalytic activity will be gone36. Besides, it was worth noting that no promoters or additives were added since the purpose of this work was to investigate the intrinsic catalytic performance of different exposed facets of χ-Fe5C2. Fe3O4@χ-Fe5C2 nanocubes and octahedra were loaded on the SiC support, denoted as Fe3O4@χ-Fe5C2 nanocubes/SiC and octahedra/SiC, respectively. The TEM images of these nanocrystals and the corresponding particle models from different orientations were shown in Supplementary Figs. 10 and 11. The catalytic properties of Fe3O4@χ-Fe5C2 nanocubes/SiC and octahedra/SiC were evaluated in a fixed-bed reactor under 20 bar of syngas (64 vol% H2, 32 vol% CO, and 4 vol% Ar) with a space velocity of 2400 mL h−1 gcat−1 at 270 °C, denoted as the standard condition. The CO conversion of Fe3O4@χ-Fe5C2 nanocubes/SiC was 45.4%, which was higher than that (21.2%) of Fe3O4@χ-Fe5C2 octahedra/SiC (Fig. 4a and Supplementary Table 3).
a CO conversion and selectivity of Fe3O4@χ-Fe5C2 nanocubes/SiC, Fe3O4@χ-Fe5C2 octahedra/SiC, and χ-Fe5C2/SiC. a refers to that the reaction was conducted under 20 bar of syngas (CO:H2 = 1:2, 2400 mL h−1 gcat−1) at 270 °C. b refers to that the reaction was conducted under 20 bar of syngas (CO:H2 = 1:2, 800 mL h−1 gcat−1) at 270 °C. b Stability tests of Fe3O4@χ-Fe5C2 nanocubes/SiC and octahedra/SiC. The reaction was conducted under 20 bar of syngas (CO:H2 = 1:2, 2400 mL h−1 gcat−1) at 270 °C. c In-situ DRIFTS spectra of Fe3O4@χ-Fe5C2 nanocubes and d Fe3O4@χ-Fe5C2 octahedra after being exposed to CO for 30 min and purged with He or H2 for 30 min at 270 °C.
To compare the catalytic activity more accurately, we calculated the TOF numbers based on the moles of CO converted per mole of surface Fe atoms per hour. The moles of Fe atoms on the surface of Fe3O4@χ-Fe5C2 nanocrystals were determined by CO pulse chemisorption measurement. The moles of Fe atoms on the surface of Fe3O4@χ-Fe5C2 nanocubes/SiC was 24.1 µmol g−1, higher than that (19.5 µmol g−1) of Fe3O4@χ-Fe5C2 octahedra/SiC (Supplementary Fig. 12a, b). The TOF number of Fe3O4@χ-Fe5C2 nanocubes/SiC was 645.9 h−1, being 1.7 times as high as that (372.7 h−1) of Fe3O4@χ-Fe5C2 octahedra/SiC. The carbon balance value of Fe3O4@χ-Fe5C2 nanocubes/SiC was 98.7%, similar to that (96.5%) of Fe3O4@χ-Fe5C2 octahedra/SiC (Supplementary Table 3). The high carbon balance value indicated that the different CO conversions were not caused by continuous carburization. For reference, we prepared pure χ-Fe5C2 nanoparticles which mainly exposed the thermodynamically most stable {510} facet through the wet-chemical route30 (Supplementary Fig. 13). The CO conversion of χ-Fe5C2 nanoparticles/SiC was 23.6% (Fig. 4a and Supplementary Table 3). As such, the {202} χ-Fe5C2 facet exposed on Fe3O4@χ-Fe5C2 nanocubes/SiC was more active than the {112} χ-Fe5C2 facet exposed on Fe3O4@χ-Fe5C2 octahedra/SiC and thermodynamically stable surfaces of iron carbides.
With respect to the selectivity, the C5+ selectivity of Fe3O4@χ-Fe5C2 nanocubes/SiC was 44.8 C%, higher than that (27.6 C%) of Fe3O4@χ-Fe5C2 octahedra/SiC (Fig. 4a and Supplementary Table 3). The C2-C4 selectivity of Fe3O4@χ-Fe5C2 nanocubes/SiC was 41.0 C%, lower than that (53.1 C%) of Fe3O4@χ-Fe5C2 octahedra/SiC (Fig. 4a and Supplementary Table 3). Additionally, the ratio of olefins to paraffins (o/p ratio) among C2-C4 for Fe3O4@χ-Fe5C2 nanocubes/SiC was 1.1, higher than that (0.6) for Fe3O4@χ-Fe5C2 octahedra/SiC (Fig. 4a and Supplementary Table 3). The selectivity for C2-C4= olefins over Fe3O4@χ-Fe5C2 nanocubes/SiC was 21.6 C%, approaching that (20.6 C%) over Fe3O4@χ-Fe5C2 octahedra/SiC (Supplementary Table 3). Additionally, the selectivity for C5–C12= olefins over Fe3O4@χ-Fe5C2 nanocubes/SiC was 17.9 C%, higher than that (11.3 C%) over Fe3O4@χ-Fe5C2 octahedra/SiC (Supplementary Table 3). As for χ-Fe5C2 nanoparticles/SiC, the selectivities for CH4, C2-C4, C5-C12, and C13+ were 15.5 C%, 48.2 C%, 34.9 C%, and 1.4 C%, respectively (Fig. 4a and Supplementary Table 3). The C2-C4= and C5-C12= selectivities were 29.8 C% and 16.9 C%, respectively (Supplementary Table 3). Actually, the distribution of hydrocarbon products for Fe3O4@χ-Fe5C2 nanocubes/SiC, Fe3O4@χ-Fe5C2 octahedra/SiC, and χ-Fe5C2 nanoparticles/SiC followed a typical Anderson-Schulz-Flory (ASF) statistics. The probabilities of chain growth (α) for Fe3O4@χ-Fe5C2 nanocubes/SiC, Fe3O4@χ-Fe5C2 octahedra/SiC, and χ-Fe5C2 nanoparticles/SiC were calculated as 0.66, 0.62, and 0.63, respectively (Supplementary Figs. 14, 15). To compare the catalytic selectivity more appropriately, we adjusted the space velocity of Fe3O4@χ-Fe5C2 octahedra/SiC to keep the reaction over cubic and octahedral nanocrystals at similar conversion levels. When the space velocity was lowered to 800 mL h−1 gcat−1, CO conversion of Fe3O4@χ-Fe5C2 octahedra/SiC reached 42.6%, approaching that (45.4%) of the cubic counterpart under the standard condition (Fig. 4a and Supplementary Table 3). The C5+ selectivity of Fe3O4@χ-Fe5C2 octahedra/SiC was 39.6 C%, while the C2-C4 hydrocarbons occupied 40.3 C% of all hydrocarbons (Fig. 4a and Supplementary Table 3). The selectivities for C2-C4= and C5-C12= were 18.3 C% and 16.0 C%, respectively (Supplementary Table 3) The α value was calculated as 0.64 at this conversion level (Supplementary Fig. 16). Therefore, the effect of crystal face on selectivity is not as great as that on activity.
We evaluated the long-term stability of Fe3O4@χ-Fe5C2 nanocrystals/SiC through a 100-h test. The CO conversion of Fe3O4@χ-Fe5C2 nanocubes/SiC fluctuated within 1% during the whole test, indicating the high stability of this catalyst (Fig. 4b). The cubic morphology was preserved after 100 h on stream (Supplementary Fig. 17a, b). We measured the thickness of the shell layer for Fe3O4@χ-Fe5C2 nanocubes after the reaction. The thickness of the shell increased from 2.0 nm to 2.6 nm after the reaction (Fig. 1c and Supplementary Fig. 17c). The lattice parameter of the Fe3O4 inner core was measured as 0.21 nm, which was indexed as the (400) facet of Fe3O4 (Supplementary Fig. 17d). The lattice parameter of the χ-Fe5C shell was 0.22 nm, which was assigned to the (202) facet of χ-Fe5C2 (Supplementary Fig. 17e). The exposed χ-Fe5C2 facets of Fe3O4@χ-Fe5C2 nanocubes were preserved after 100 h on stream. The EELS image implied that the core region mainly comprised Fe and O elements while the shell region contained Fe and C elements (Supplementary Fig. 17f ). We also conducted Mössbauer spectroscopy to characterize the compositions of the iron phase in the used Fe3O4@χ-Fe5C2 nanocubes after 100 h on stream (Supplementary Fig. 18a). The content of χ-Fe5C2 increased from 33.2% to 39.8% after the reaction (Supplementary Table 4).
In contrast, the CO conversion of Fe3O4@χ-Fe5C2 octahedra/SiC declined continuously. The conversion was only 11.7% after 100 h, about half of that (20.1%) at the beginning (Fig. 4b). To investigate the reason for the deactivation, we characterized Fe3O4@χ-Fe5C2 octahedra/SiC after 100 h on stream. As shown in Supplementary Fig. 19a, b, the solid octahedra collapsed after the reaction. The formation of Fe3O4@χ-Fe5C2 octahedra with multiple voids was ascribed to the Kirkendall effect37,38,39. Specifically, as the blockage of active sites by long-chain hydrocarbons and non-graphitic carbon, Fe3O4@χ-Fe5C2 octahedra were gradually deactivated. Meanwhile, the carbon chemical potential of reaction conditions changed. The dynamic balance of the hydrocarbon production, surface oxidation, and carburization in the syngas environment was broken. This led to the diffusion of Fe atoms between the Fe3O4 core and χ-Fe5C2 shell. The void formation in Fe3O4@χ-Fe5C2 octahedra due to differential diffusion rates of Fe atoms. The thickness of the shell increased from 1.7 nm to 3.5 nm after the reaction (Fig. 3c and Supplementary Fig. 19c). The lattice parameters of the inner core and the outer shell were measured as 0.48 nm and 0.21 nm, which were assigned to the (111) facet of Fe3O4 and (112) facet of χ-Fe5C2, respectively (Supplementary Fig. 19d, e). The exposed χ-Fe5C facet of Fe3O4@χ-Fe5C2 octahedra was preserved after the reaction. The distribution of Fe3O4 at the core and χ-Fe5C2 at the shell was supported by the EELS image (Supplementary Fig. 19f). The content of χ-Fe5C2 in Fe3O4@χ-Fe5C2 octahedra increased from 29.5% to 40.4% after 100 h on stream (Supplementary Fig. 18b and Supplementary Table 4). The thickness of the shell layer for both Fe3O4@χ-Fe5C2 nanocubes and octahedra increased after the reaction. Therefore, the stabilities of Fe3O4@χ-Fe5C2 nanocrystals/SiC were also affected by the exposed facets.
To investigate the textural properties, we carried out N2 physisorption characterizations. The pore-diameter distributions were analyzed using the Barrett-Joyner-Halenda (BJH) method. As shown in (Supplementary Fig. 4c, d), the surface layer of both Fe3O4@χ-Fe5C2 nanocubes and octahedra were not porous. The absence of pores at the surface layer was also confirmed by HAADF-STEM images (Figs. 1b and 3b). We also measured the textural properties of spent Fe3O4@χ-Fe5C2 nanocubes and octahedra after 100 h on stream (Supplementary Fig. 20a, b). The surface layer of spent Fe3O4@χ-Fe5C2 nanocubes remained nonporous after 100 h on stream (Supplementary Fig. 20c). In contrast, spent Fe3O4@χ-Fe5C2 octahedra contained mesopores as revealed by the pore-diameter distribution and HAADF-STEM image (Supplementary Fig. 20d). The average mesopore diameter was determined as 16.0 nm by the BJH method (Supplementary Fig. 20d).
To investigate other underlying mechanisms for catalyst deactivation, we employed Raman spectroscopy to analyze the surfaces of Fe3O4@χ-Fe5C2 nanocubes and octahedra after 100 h. The presence of peaks at 1330 cm−1 indicated the existence of disordered carbon (D band), while those at 1592 cm−1 signified graphite (G band) (Supplementary Fig. 21a, b). Significantly higher intensities of these peaks were observed on the surface of spent Fe3O4@χ-Fe5C2 octahedra compared with spent nanocubes, suggesting a greater accumulation of deposited carbon on the octahedral surface (Supplementary Fig. 21a, b). For a more precise comparison, we conducted thermogravimetric analysis (TGA) under the N2 atmosphere on both Fe3O4@χ-Fe5C2 nanocubes and octahedra after the reaction. The weight loss between 200 and 500 °C was attributed to the removal of long-chain hydrocarbons from the surface, while the weight loss beyond 500 °C was associated with the loss of non-graphitic carbon. In the case of spent Fe3O4@χ-Fe5C2 nanocubes, a weight loss of 6.2 wt% was observed (Supplementary Fig. 21c). Conversely, during TGA testing, spent Fe3O4@χ-Fe5C2 octahedra exhibited a total weight loss of 13.5 wt% due to long-chain hydrocarbons and deposited carbon (Supplementary Fig. 21d). The higher residual weight of long-chain hydrocarbons and deposited carbon on the octahedral structure suggests a more pronounced blockage of active sites compared to nanocubes. Hence, we hypothesize that carbon deposition also contributes to the deactivation of Fe3O4@χ-Fe5C2 octahedra.
Mechanistic insights into the facet effect
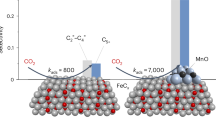
To rationalize the facet-dependent FTS activity, we explored CO dissociation pathways by conducting in-situ diffuse reflection infrared spectroscopy (DRIFTS) measurements at 270 °C. When Fe3O4@χ-Fe5C2 nanocubes were exposed to 1 bar of CO and purged with He, three sets of peaks were observed (Fig. 4c). The peaks at 2173 and 2115 cm−1 were assigned to the gaseous CO, while the peak at 2058 cm−1 arose from the stretching vibration of linearly adsorbed CO19,40,41,42. The peaks at 2360 and 2336 cm−1 corresponded to the gaseous CO2, indicating the direct dissociation of CO on Fe3O4@χ-Fe5C2 nanocubes. As for the exposure to CO and purging with H2, other sets of peaks emerged besides the peaks for CO and gaseous CO2 (Fig. 4c). Specifically, the peak at 2955 cm−1 derived from the asymmetrical stretching vibration of C-H in CH3* (refs. 43,44). The peaks at 2919 and 2849 cm−1 were indexed as the asymmetrical and symmetrical stretching vibrations of C-H in CHO* and CH2*, respectively45,46. Notably, the peak at 1741 cm−1 was ascribed to the stretching vibration of C = O in CHO* species, which indicated the existence of a hydrogen-assisted dissociation route47,48. For Fe3O4@χ-Fe5C2 octahedra, when the sample was exposed to CO and purged with He, the peaks for gaseous CO2 appeared (Fig. 4d). As for the treatment with CO and purging with H2, the in-situ DRIFTS profile showed the peaks for CH3*, CH2*, CO2, and CO, in the absence of CHO* or COH* (Fig. 4d). We also conducted in-situ DRIFTS experiments under 20 bar of syngas, simulating realistic reaction environments (Supplementary Fig. 22, Supplementary Table 5). The appearance of gaseous CO2 peaks at 2360 and 2336 cm−1 provided evidence for direct dissociation occurring on both Fe3O4@χ-Fe5C2 nanocubes and octahedra (Supplementary Fig. 22, Supplementary Table 5). Moreover, the presence of CHO* species, as indicated by a peak at 1741 cm−1, was observed solely on the Fe3O4@χ-Fe5C2 nanocubes catalyst and absent on the octahedral counterpart (Supplementary Fig. 22, Supplementary Table 5). Therefore, Fe3O4@χ-Fe5C2 octahedra enabled the direct dissociation of CO, while both direct and hydrogen-assisted CO dissociation routes existed on Fe3O4@χ-Fe5C2 nanocubes (Supplementary Fig. 23).
To investigate the activation energy of different CO dissociation routes, we plotted the area of linearly adsorbed CO peak at 2058 cm−1 as a function of time at 190, 230, and 270 °C (Supplementary Figs. 24–27). Based on the slope of the decrease in peak area with purge time, the rate of CO dissociation (k) was obtained. Activation energies of CO dissociation were calculated with the Arrhenius equation7. For Fe3O4@χ-Fe5C2 nanocubes, the activation energy for hydrogen-assisted dissociation of CO was 68.6 kJ mol−1, significantly lower than that (103.7 kJ mol−1) direct dissociation of CO (Supplementary Fig. 28a). With regard to Fe3O4@χ-Fe5C2 octahedra, the activation energy (83.2 kJ mol−1) of CO dissociation with H2 approximated to that (80.4 kJ mol−1) without H2 (Supplementary Fig. 28b). Therefore, Fe3O4@χ-Fe5C2 nanocrystals exhibited facet-dependent FTS activities derived from the alteration of reaction paths which changed the activation energy. Specially, Fe3O4@χ-Fe5C2 nanocubes followed hydrogen-assisted CO dissociation which lowered the activation energy, relative to that of direct CO dissociation over Fe3O4@χ-Fe5C2 octahedra.
We conducted DFT calculations to rationalize CO direct dissociation route and hydrogen-assisted dissociation path on χ-Fe5C2(202) and χ-Fe5C2(112) surface. For χ-Fe5C2(202) surface, the hydrogen-assisted CO dissociation route is the dominating route, since its energy barrier of the rate-limiting step (CO* + H* → HCO*) 1.23 eV is much lower than the direct CO dissociation route with an energy barrier as high as 2.85 eV (Supplementary Figs. 29–32). For χ-Fe5C2(112) surface, the direct CO dissociation route exhibited a lower energy barrier (1.37 eV) than the hydrogen-assisted CO dissociation route (1.54 eV), implying the direct dissociation as the main route of CO dissociation (Supplementary Figs. 33–36). Besides, the energy barrier of the hydrogen-assisted CO dissociation route on the χ-Fe5C2(202) surface is lower than that of the CO direct dissociation on the χ-Fe5C2(112) surface (Supplementary Figs. 32 and 35). The DFT conclusion was consistent with in-situ DRIFTS spectra result.
We explored the adsorption of CO and H2 by conducting pulse chemisorption measurements to rationalize why Fe3O4@χ-Fe5C2 nanocubes/SiC exhibited better carbon-chain growth ability. The amount of adsorbed gas was calculated on the difference between the total amount of gas injected and the amount measured at the outlet from the sample. The amount of adsorbed CO over Fe3O4@χ-Fe5C2 nanocubes/SiC was 24.1 µmol g−1, higher than that (19.5 µmol g−1) of Fe3O4@χ-Fe5C2 octahedra (Supplementary Fig. 12). While the amount of adsorbed H2 over Fe3O4@χ-Fe5C2 nanocubes/SiC was 7.3 µmol g−1, lower than that (11.0 µmol g−1) of the octahedral counterpart (Supplementary Fig. 37). The results indicated that Fe3O4@χ-Fe5C2 nanocubes/SiC improved the CO adsorption and suppressed the H2 adsorption compared to the octahedral counterpart. Thus, the surface CO/H2 ratio of Fe3O4@χ-Fe5C2 nanocubes/SiC was 3.3, higher than that (1.8) of the octahedral counterpart (Supplementary Fig. 38). With the increase of the surface CO/H2 ratio, the CH4 production over Fe3O4@χ-Fe5C2 nanocubes/SiC was suppressed, while hydrocarbon products shifted to long-chain hydrocarbons. We also conducted DFT calculations to investigate the facet effect on CH4 production. The CH2* + H* energy barrier of χ-Fe5C2(202) is 1.03 eV, higher than that (0.70 eV) of χ-Fe5C2(112) (Supplementary Figs. 39–41). The energy barrier of the CH2* + CH2* step over χ-Fe5C2(202) facet is 0.70 eV, lower than that (1.03 eV) of CH2* + H*. As for χ-Fe5C2(112) facet, the energy barrier of the CH2* + CH2* step is 0.60 eV, approaching to that (0.7 eV) of CH2* + H*. Thus, compared with Fe3O4@χ-Fe5C2 nanocubes, the octahedral counterpart benefits the CH4 production.
We demonstrated the dependence of catalytic performance on the exposed facet of iron carbides in FTS. Uniformly exposed {202} and {112} facets of χ-Fe5C2 were successfully fabricated as the model system. With the help of well-defined catalysts, we identified the intrinsically active, selective, and stable facets of χ-Fe5C2. Our findings deepen the understanding of Fe-based FTS catalysts from the phase and size to the facet. In addition, this work also provides a facile method to precisely control the exposed surfaces of iron carbides for both future fundamental studies and practical applications.
Methods
Chemicals and materials
Oleylamine (OAm, >70%), oleic acid (OA, 90%), benzyl ether (BE, 99%), iron(III) acetylacetonate (Fe(acac)3, >99.9%), and 4-biphenylcarboxylic acid (99%) were obtained from Sigma-Aldrich. SiC (β-phase, 99.8%) was obtained from Adamas-beta®. All other chemicals were analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water with a resistivity of 18.2 MΩ cm was used for the preparation of all aqueous solutions (Milli-Q®).
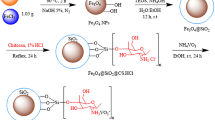
Synthesis of Fe3O4 nanocrystals
In a typical synthesis of Fe3O4 nanocubes, Fe(acac)3 (1.4 g) and 4-biphenylcarboxylic acid (1.0 g) were dissolved in a mixture of BE (20.0 mL) and OA (2.5 mL). The solution was degassed at 120 °C for 30 min. Subsequently, the solution was heated to 290 °C at 10 °C min−1 and kept for 30 min with a stirring rate of 300 rpm. As for the synthesis of Fe3O4 octahedra, Fe(acac)3 (1.0 g) was dissolved in a mixture of BE (20.0 mL), OAm (2.3 mL), and OA (1.6 mL), followed by degassing at 120 °C for 30 min. The solution was heated to 220 °C at 20 °C min−1 with a stirring rate of 300 rpm and kept for 1 h. Afterward, the solution was heated to 300 °C at 20 °C min−1 and kept for 2 h. After the solution had been cooled down to room temperature, the products were precipitated by ethanol, washed three times with hexane, and re-dispersed in hexane.
Synthesis of Fe3O4@χ-Fe5C2 nanocrystals
For the synthesis of Fe3O4@χ-Fe5C2 nanocrystals, as-prepared Fe3O4 nanocrystals (50 mg) were loaded into a fixed-bed reactor with an inner diameter of 9 mm. Fe3O4 nanocrystals were reduced in H2 under 1 bar with a gas-flow rate of 100 mL min−1 at 270 °C for 10 h, while the heating ramp was 1 °C min−1. Afterwards, the obtained samples underwent surface reconstruction under 20 bar of syngas (32 vol% H2, 64 vol% CO, and 4 vol% Ar) with a space velocity of 2400 mL h−1 gcat−1 at 270 °C for 20 h. Considering that the iron carbides were highly sensitive to the atmosphere, we used inert gas to protect and store the catalyst after reaction. Specifically, we switched the feed gas to N2 when the reaction was stopped. The reaction tubes were sealed via valves at both ends after cooling the reactor to room temperature. Afterwards, the reaction tubes were transferred to a N2-filled glove box. The samples were stored in the glove box before characterizations.
Synthesis of pure χ-Fe5C2 nanocrystals
Octadecylamine of 14.5 g and cetyl trimethyl ammonium bromide (CTAB) of 0.113 g were mixed in a four-neck flask under stirring and degassed under the N2 flow, followed by being heated to 120 °C. Afterwards, Fe(CO)5 (3.6 mmol) was injected into the mixture under the N2 blanket. The mixture was heated to 180 °C at 10 °C min−1 and kept at this temperature for 10 min. Subsequently, the mixture was further heated to 350 °C at 10 °C min−1 and kept at this temperature for 10 min. After being cooled to room temperature, the products were washed with a mixture of ethanol and hexane.
Mössbauer measurements
57Fe Mössbauer spectra were carried out on a Topologic 500 A spectrometer driving with a proportional counter at room temperature. The radioactive source was 57Co (Rh) moving in a constant acceleration mode. Data analyses were performed assuming a Lorentzian lineshape for computer folding and fitting.
Catalytic tests
The Fischer-Tropsch reaction was carried out in a fixed-bed reactor under 20 bar of syngas at 270 °C. Generally, Fe3O4@χ-Fe5C2 nanocrystals (50 mg, 20–40 meshes) were diluted with SiC (450 mg, 20–40 meshes). The sample was loaded into a fixed-bed reactor with an inner diameter of 9 mm. Subsequently, a mixture including 96 vol% of H2/CO mixed gas (64 vol% H2, 32 vol% CO) and 4 vol% of Ar (as an internal standard) was introduced to the reactor as the feeding gas at a space velocity of 2400 mL h−1 gcat−1.
The gaseous products were monitored by online gas chromatographs (Shimadzu GC-2014). An ice trap with 2.0 g of solvent (n-dodecane) was employed to capture the liquid hydrocarbons in the effluent. The liquid products with 0.1 g of an internal standard (decalin) were analyzed using an offline Shimadzu GC-2014.
CO conversion was calculated as follows:
where COinlet and COoutlet are moles of CO at the inlet and outlet, respectively.
CO2 selectivity was calculated according to:
where CO2 outlet refers to moles of CO2 at the outlet.
The selectivity of hydrocarbon CnHm was obtained according to:
where CnHm outlet represents moles of individual hydrocarbon product at the outlet.
Carbon balance was calculated according to:
where CnHm and CO2 represent the moles of the produced hydrocarbons and CO2, respectively. COinlet and COoutlet are moles of CO at the inlet and outlet, respectively. The carbon balances were over 95.0%.
In-situ DRIFTS measurements after different gas treatments
In-situ DRIFTS experiments were conducted in an elevated-pressure cell (DiffusIR Accessory PN 041-10XX) with a Fourier transform infrared spectrometer (TENSOR II Sample Compartment RT-DLaTGS) and a liquid-nitrogen-cooled MCT detector. The outlet gas was analyzed by a mass spectrometer (Hiden HPR20). Spectra were measured accumulating 64 scans at a resolution of 4 cm−1. Prior to the test, the samples were cleaned in He with a gas-flow rate of 100 mL min−1 at 270 °C for 1 h. Then the samples were exposed to 1 bar of CO for 30 min and subsequently purged by He or H2 for 30 min at 270 °C. As for the in-situ DRIFTS measurements at different temperatures, the background spectra of the samples were acquired under He after the temperature of the samples dropped to a specified temperature. Then, the samples were exposed to 1 bar of CO for 30 min and subsequently purged with He or H2 at a specified temperature. As for the in-situ DRIFTS experiments under 20 bar of syngas, thorough cleaning of the samples in He at 270 °C for 1 h ensured the elimination of any contaminants. Background spectra were acquired under He flow, followed by exposure to a 20-bar syngas mixture (CO/H2) at 270 °C for 30 min.
DFT methods
Spin-polarized DFT calculations were conducted using the Vienna ab initio simulation package (VASP)49. The projector-augmented wave method and the Perdew-Burke-Ernzerhof functional were implemented in the code50. Fe5C2(202) and Fe5C2(112) slab were established with the same stoichiometric atoms (Fe60C24). A plane-wave basis set with a cutoff energy of 400 eV was used with the K-points of 3 × 3 × 1 and vacuum thickness of 15 Å. During the structure optimization, the two layers of atoms at the bottom were fixed, while the others include the adsorbates were relaxed with the electronic convergence of 0.02 eV/Å. For each elementary step, the initial states and final states are firstly optimized. The transition states are searched using the climbing image nudged elastic band method (CI-NEB) and confirmed by the vibrational frequencies analysis51.
CO pulse chemisorption measurements
CO pulse chemisorption measurements were performed using a Micromeritics Autochem 2920 chemisorption analyzer with an active loop volume of 0.1 mL. In a typical measurement, 100 mg of Fe3O4@χ-Fe5C2 nanocrystals/SiC were packed into a reactor with a quartz tube. Prior to the test, the samples were cleaned in He with a gas-flow rate of 100 mL min−1 at 270 °C for 5 h. After cooling down to 50 °C under He flow, CO/He pulses (10 vol% CO and 90 vol% He) were injected until adsorption reached saturation. The amount of adsorbed CO was calculated on the difference between the total amount of CO injected and the amount measured at the outlet from the sample. The metal dispersion was calculated by assuming the ratio of CO to surface metal atom as 1:1.
TOF number was calculated according to:
H2 pulse chemisorption measurements
H2 pulse chemisorption measurements were performed using a Micromeritics Autochem 2920 chemisorption analyzer with an active loop volume of 0.1 mL. In a typical measurement, 100 mg of Fe3O4@χ-Fe5C2 nanocrystals/SiC were packed into a reactor with a quartz tube. Prior to the test, the samples were cleaned in He with a gas-flow rate of 100 mL min−1 at 270 °C for 5 h. After cooling down to 50 °C under He flow, H2/Ar pulses (10 vol% H2 and 90 vol% Ar) were injected until adsorption reached saturation. The amount of adsorbed H2 was calculated on the difference between the total amount of H2 injected and the amount measured at the outlet from the sample.
Instrumentations
TEM images were taken using a Hitachi H-7700 transmission electron microscope at an acceleration voltage of 100 kV. HAADF and EDS analysis were collected on a JEOL ARM-200F field-emission transmission electron microscope operating at 200 kV accelerating voltage. XPS measurements were conducted on an ESCALAB 250 (Thermo-VG Scientific, USA) with an Al Kα X-ray source (1486.6 eV protons) in Constant Analyzer Energy (CAE) mode with a pass energy of 30 eV for all spectra. XRD characterization was performed using a Philips X’Pert Pro X-ray diffractometer with a monochromatized Cu Kα radiation source and a wavelength of 0.1542 nm. BET measurements were carried out on Micromeritics AutoChem II 2020. TGA spectra were conducted on Pyris Diamond TG-DTG. Raman spectra were detected by a Renishaw RM3000 Micro-Raman system with a 514.5 nm Ar laser.
Data availability
The data generated in this study are provided in the Supplementary Information.
References
Van Santen, R. A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 42, 57–66 (2009).
Torres Galvis, H. M. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 335, 835–838 (2012).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Xu, Y. et al. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products. Science 371, 610–613 (2021).
Fang, W. et al. Physical mixing of a catalyst and a hydrophobic polymer promotes CO hydrogenation through dehydration. Science 377, 406–410 (2022).
Jiao, F. et al. Disentangling the activity-selectivity tradeoff in catalytic conversion of syngas to light olefins. Science 380, 727–730 (2023).
Tuxen, A. et al. Size-dependent dissociation of carbon monoxide on cobalt nanoparticles. J. Am. Chem. Soc. 135, 2273–2278 (2013).
Zhong, L. et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 538, 84–87 (2016).
Li, W. et al. Chemical insights into the design and development of face-centered cubic ruthenium catalysts for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 139, 2267–2276 (2017).
Li, J. et al. Integrated tuneable synthesis of liquid fuels via Fischer-Tropsch technology. Nat. Catal. 1, 787–793 (2018).
Cheng, Q. et al. Confined small-sized cobalt catalysts stimulate carbon-chain growth reversely by modifying ASF law of Fischer-Tropsch synthesis. Nat. Commun. 9, 3250 (2018).
Sun, X. et al. Manufacture of highly loaded silica-supported cobalt Fischer-Tropsch catalysts from a metal organic framework. Nat. Commun. 8, 1680 (2018).
Zhang, Y. et al. Tuning reactivity of Fischer-Tropsch synthesis by regulating TiOx overlayer over Ru/TiO2 nanocatalysts. Nat. Commun. 11, 3185 (2020).
de Smit, E. & Weckhuysen, B. M. The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 37, 2758–2781 (2008).
Xu, K. et al. ε-Iron carbide as a low-temperature Fischer-Tropsch synthesis catalyst. Nat. Commun. 5, 583 (2014).
Santos, V. P. et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 6, 6451 (2015).
Yao, S. et al. Reconstruction of the wet chemical synthesis process: the case of Fe5C2 nanoparticles. J. Phys. Chem. C 121, 5154–5160 (2017).
Yang, C. et al. Construction of synergistic Fe5C2/Co heterostructured nanoparticles as an enhanced low temperature Fischer-Tropsch synthesis catalyst. ACS Catal. 7, 5661–5667 (2017).
An, B. et al. Pyrolysis of metal-organic frameworks to Fe3O4@Fe5C2 core-shell nanoparticles for Fischer-Tropsch synthesis. ACS Catal. 6, 3610–3618 (2016).
Ding, M. et al. Study on reduction and carburization behaviors of iron phases for iron-based Fischer-Tropsch synthesis catalyst. Appl. Energy 160, 982–989 (2015).
de Smit, E. et al. Stability and reactivity of ε-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: controlling µc. J. Am. Chem. Soc. 132, 14928–14941 (2010).
Fu, D. et al. Probing the structure evolution of iron-based Fischer-Tropsch to produce olefins by Operando Raman spectroscopy. ChemCatChem. 7, 752–756 (2015).
Torres Galvis, H. M. et al. Iron particle size effects for direct production of lower olefins from synthesis gas. J. Am. Chem. Soc. 134, 16207–16215 (2012).
Wang, P. et al. Synthesis of stable and low-CO2 selective ε-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 4, eaau2947 (2018).
Lyu, S. et al. Stabilization of ε-iron carbide as high-temperature catalyst under realistic Fischer-Tropsch synthesis conditions. Nat. Commun. 11, 6219 (2020).
Zhao, H. et al. Synthesis of iron-carbide nanoparticles: identification of the active phase and mechanism of Fe-based Fischer-Tropsch synthesis. CCS Chem. 2, 2712–2724 (2021).
Chen, W. et al. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts. Catal. Today 311, 8–22 (2018).
Chen, B. et al. Charge-tuned CO activation over a χ‑Fe5C2 Fischer-Tropsch catalyst. ACS Catal. 8, 2709–2714 (2018).
Guo, W. et al. Visualization of on-surface ethylene polymerization through ethylene insertion. Science 375, 1188–1191 (2022).
Yang, C., Zhao, H., Hou, Y. & Ma, D. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 134, 15814–15821 (2012).
Wang, J. et al. Elucidating surface and bulk phase transformation in Fischer-Tropsch synthesis catalysts and their influences on catalytic performance. ACS Catal. 9, 7976–7983 (2019).
Liu, J. & Zhang, J. Nanointerface chemistry: lattice-mismatch-directed synthesis and application of hybrid nanocrystals. Chem. Rev. 120, 2123–2170 (2020).
Zhu, J. et al. Dynamic structural evolution of iron catalysts involving competitive oxidation and carburization during CO2 hydrogenation. Sci. Adv. 8, eabm3629 (2022).
Figuerola, A. et al. Epitaxial CdSe-Au nanocrystal heterostructures by thermal annealing. Nano Lett. 10, 3028–3036 (2010).
Weng, W. et al. Twin-mediated epitaxial growth of highly lattice-mismatched Cu/Ag core-shell nanowires. Nanoscale 10, 9862–9866 (2018).
Gilroy, K., Ruditskiy, A., Peng, H., Qin, D. & Xia, Y. Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116, 10414–10472 (2016).
Li, A. et al. Rational design of yolk-shell nanostructures for photocatalysis. Chem. Soc. Rev. 48, 1874 (2019).
Starchikov, S. S. et al. Evolution of the phase composition, crystal structure and magnetic properties of core@shell nanoparticles obtained during conversion of ferrocene at high pressure and high temperature. Appl. Surf. Sci. 615, 156269 (2023).
LaGrow, AlecP. et al. Environmental STEM study of the oxidation mechanism for iron and iron carbide nanoparticles. Materials 15, 1557 (2022).
Liu, R. et al. Identifying correlations in Fischer-Tropsch synthesis and CO2 hydrogenation over Fe-based ZSM-5 catalysts. J. CO2 Util. 41, 101290 (2020).
Kumar, N., Jothimurugesan, K., Stanley, G., Schwartz, V. & Spivey, J. In situ FT-IR study on the effect of cobalt precursors on CO adsorption behavior. J. Phys. Chem. C 115, 990–998 (2010).
Hexana, W. & Coville, N. Indium as a chemical promoter in Fe-based Fischer-Tropsch synthesis. Appl. Catal. A 377, 150–157 (2010).
McNab, A., McCue, A., Dionisi, D. & Anderson, J. Quantification and qualification by in-situ FTIR of species formed on supported-cobalt catalysts during the Fischer-Tropsch reaction. J. Catal. 353, 286–294 (2017).
Huynh, H. L. et al. Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J. Catal. 392, 266–277 (2020).
Han, X. et al. Interface-induced phase evolution and spatial distribution of Fe-based catalysts for Fischer-Tropsch synthesis. ACS Catal. 13, 6525–6535 (2023).
Bahri, S., Pathak, S. & Upadhyayula, S. Transient HCO/HCOO− species formation during Fischer-Tropsch over an Fe-Co spinel using low Ribblet ratio syngas: a combined operando IR and kinetic study. Sustain. Energy Fuels 7, 708–726 (2023).
Miao, B., Ma, S. S. K., Wang, X., Su, H. & Chan, S. H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 6, 4048–4058 (2016).
Hartman, T., Geitenbeek, R., Whiting, G. & Weckhuysen, B. Operando monitoring of temperature and active species at the single catalyst particle level. Nat. Catal. 2, 986–996 (2019).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396–1396 (1997).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by National Key Research and Development Program of China (2023YFA1508003 to H.L., 2021YFA1500500 to J.Z., 2019YFA0405600 to J.Z.), CAS Project for Young Scientists in Basic Research (YSBR-051 to J.Z.), National Science Fund for Distinguished Young Scholars (21925204 to J.Z.), NSFC (22221003 to J.Z., 22250007 to J.Z., 22361162655 to J.Z., 22204158 to W.W., 22308346 to H.L.), Fundamental Research Funds for the Central Universities, Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP004 to J.Z.), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (YLU-DNL Fund 2022012 to J.Z.), USTC Research Funds of the Double First-Class Initiative (YD9990002014 to H.L.), Joint Funds from the Hefei National Synchrotron Radiation Laboratory (KY2340000157 to W.W., KY9990000202 to H.L.), the DNL Cooperation Fund, CAS (DNL202003 to J.Z.), and International Partnership Program of Chinese Academy of Sciences (123GJHZ2022101GC to J.Z.). J.Z. acknowledges support from the Tencent Foundation through the XPLORER PRIZE. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. This work was also partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication.
Author information
Authors and Affiliations
Contributions
W.W. and J.L. equally contributed to this work. W.W., J.L., H.L., and J. Zeng designed the studies and wrote the paper. W.W., J.L., and L.L. synthesized catalysts. W.W., J.L., B.H., and H.L. performed catalytic tests. W.W. and W.M. conducted characterizations. J. Zhao conducted DFT calculations. S.H. and C.M. conducted HAADF-STEM characterizations. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Noritatsu Tsubaki and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Luo, J., Zhao, J. et al. Facet sensitivity of iron carbides in Fischer-Tropsch synthesis. Nat Commun 15, 6108 (2024). https://doi.org/10.1038/s41467-024-50544-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50544-1
This article is cited by
-
NiFe2O4 spinel engineering for transcending the dilemma of activity–selectivity in CO2 hydrogenation to ethanol
Nature Communications (2025)