Abstract
The plant microbiota can complement host functioning, leading to improved growth and health under unfavorable conditions. Microbiome engineering could therefore become a transformative technique for crop production. Microbiome genes, abbreviated as M genes, provide valuable targets for shaping plant-associated microbial communities.
Similar content being viewed by others
New strategies are needed to secure global crop production
Agriculture currently relies on high inputs of agrochemicals to maintain crop production. This has not only led to polluted environments and health issues, but also contributed to the crossing of planetary boundaries1. Various global initiatives, such as the European Green Deal, aim at substantially reducing agrochemical inputs during the next years. The implementation of such initiatives is currently mainly hindered by the fact that we lack viable strategies that would allow us to maintain the required crop production without chemical plant protection. Plant breeding focusing on resistance genes (R genes) and susceptibility genes (S genes) has emerged as a promising solution to develop crop plants that are less susceptible to pathogens. However, such approaches, especially those based on R genes, can only provide short-term solutions as diverse and rapidly evolving pathogens can overcome them2.
Microbiome studies, which have been carried out intensively over the last 15 years, have contributed to a better understanding of plant health and disease. They have also provided support for the holobiont theory. This theory is based on the assumption that microbial communities together with multicellular hosts can result in various phenotypes, offering extended possibilities for adaptation in various environments. In rice, it was demonstrated that the presence of a specific seed-endophytic bacterium can shape an entirely disease-resistant plant phenotype3. A high number of studies conducted during the last decades has provided evidence that various microorganisms have plant-beneficial traits when naturally present or artificially introduced. Introduced biological agents often result in significant plant growth promotion and disease protection. However, there are substantial limitations in terms of their applicability due to frequently occurring inefficacy or low efficacy under certain field conditions4. Competition with the local microbiota, which can vary between hosts and environments, is one of the unpredictable factors that must be accounted for when living organisms are used for plant protection.
Significant public and private investments are currently being made to support the development of technologies that manipulate the host microbiota as a whole, also known as ‘microbiome engineering’. Such technologies hold more promise than the currently available ones based on the application single strains or defined microbial consortia. One general strategy to engineer microbiomes is to alter traits of the host. A recent review article highlights the potential of plant microbiome engineering, but the authors also point out that we currently lack detailed knowledge related to specific host genes that are involved in directed assembly of microbial communities with desired functions5. The identification of such genes will play a pivotal role in enabling targeted modifications of the plant microbiome.
Engineering crop microbiomes via host genetics
Studies conducted during the last years have shown that plants can recruit specific microbes via their metabolites6,7,8. The mechanisms that were discovered so far are needed for targeted recruitment of bacteria as well as fungi8,9. Such findings provide new options to harness specific host genetic traits to shape and maintain a microbiota with desirable functioning. Knowledge about microbiome-regulating host genes could be harnessed for the generation of genetically modified plants and more importantly to specifically screen for natural variation in these genes. The latter will likely result in targeted breeding approaches that will face less restrictions in agricultural applications as compared to genetically modified plants. The overall approach was introduced as M gene breeding by Su and colleagues8. Observations made in this study indicate that specific M gene haplotypes in rice plants can significantly enrich specific microbiome components resulting in increased protection against pathogens. This could be further exploited in targeted breeding approaches. Implementation of M genes in plant breeding has the potential to provide long-term protection against phytopathogens. This is mainly supported by microbial diversity being accompanied by chemical diversity due to a wide range of metabolites that can be produced by different members of the microbiota. Pathogens would therefore have to develop resistances against a diverse set of bioactive compounds that are synthesized by M gene-enriched microbes. This is in contrast to the direct effect of certain plant metabolites against pathogens, which can more readily result in resistance development. In addition, knowledge about M gene activity can likely be harnessed to bidirectionally optimize plant-microbe interactions that result in improved plant growth or productivity10,11. In all the examples described, it will be important to take potential adaptations of plants to local environments and their microbiomes into consideration10. It is also noteworthy to mention that it is currently unclear if certain plant pathogens also respond to specific M genes, which would allow them to potentially hijack certain mechanisms for their own benefit.
Various genes involved in the biosynthesis or regulation of plant metabolites known to shape the microbiota are promising targets for M gene breeding. Distinct lignin precursors, and especially p-coumaric acid, regulate bacterial community structures in the phyllosphere and endosphere of plants8,12. Scopoletin, a coumarin that branches out of lignin biosynthesis, was shown to selectively enrich plant-beneficial microorganisms in the rhizosphere13. Several other plant coumarins are also known for their effects on the plant microbiome7. Targeted plant breeding to optimize the production of microbiome-shaping compounds could result in less susceptibility to phytopathogens and increased plant productivity. This has the potential to substantially lower the global use of chemical pesticides and fertilizers. A growing knowledge base about plant genes that are in control of the microbiome will pave the way for such approaches14,15. Obstacles that will be likely encountered are related to multiple different M genes and exometabolites that are sometimes shaping the microbiome in a concerted way16,17. This will increase the complexity related to the implementation of M gene breeding approaches in such cases but is not an impassable hurdle if the connected genes in plants are known.
Further considerations related to M gene breeding
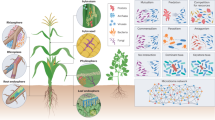
The fundamental basis for M gene breeding is readily available, meaning that the first attempts can already be made. Screening for M gene activity can be implemented as part of pre-breeding approaches where available germplasm material is utilized. Targeted approaches can also be implemented to further improve elite plant varieties by fine-tuning their M genes into a desired direction. When designing studies to identify novel M genes, distinct particularities of plant microbiomes should be taken into account. Intra-species genetic diversity provides a highly suitable basis for the identification of novel M genes by allowing to search for microbiome associations at single-nucleotide polymorphism (SNP) level (Fig. 1)8. Future identification of M genes will be facilitated if plants subjected to microbiome profiling are obtained from the same location and growth stage, as environmental and host-specific factors could obscure their implications in shaping microbial communities. In addition, upcoming genome-wide association studies (GWAS) targeting microbiomes would generally benefit from relying on shotgun-sequenced metagenomes; currently they are mostly based on marker gene amplicons that are more prone to bias15. Underutilized crops, also known as orphan crops, could be a valuable resource to study co-evolved associations between plants and their microbiota. This is especially due to their generally better adaptation to local environments, where also specialized microbial communities are more likely to be found. To enable such approaches, suitable genetic resources must be generated18. Such resources are currently scarce, however, the importance of intensifying research efforts to generate them was recognized19.
Specific host genes are associated with the structure and composition of the microbiome. Single-nucleotide polymorphisms (SNPs) in these genes can lead to alterations of the microbiome. Knowledge about SNPs associated with the whole microbiota, or distinct components of it, provides the basis for targeted M gene breeding approaches. Screening for specific M gene haplotypes connected with desirable microbiome structures can be implemented as part of pre-breeding strategies with germplasm collections. If successfully implemented, crop plants with an optimized microbiome will be less reliant on chemical pesticides and fertilizers.
The more associations are identified between plant genes and microbiome components, the more likely it is that these findings will lead to practical applications. This can be accelerated if targeted approaches are implemented in plant microbiome research. If it proves viable, M gene breeding not only can increase sustainability in agriculture but also contribute to global food security.
References
Richardson, K. et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 9, eadh2458 (2023).
Stam, R. & McDonald, B. A. When resistance gene pyramids are not durable—the role of pathogen diversity. Mol. Plant Pathol. 19, 521 (2018).
Matsumoto, H. et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 7, 60–72 (2021).
Jurburg, S. D. et al. Potential of microbiome-based solutions for agrifood systems. Nat. Food 3, 557–560 (2022).
Zhan, C., Matsumoto, H., Liu, Y. & Wang, M. Pathways to engineering the phyllosphere microbiome for sustainable crop production. Nat. Food 3, 997–1004 (2022).
O’Banion, B. S. et al. Plant inositol transport influences bacterial colonization phenotypes. Curr. Biol. 33, 3111–3124 (2023).
Stringlis, I. A., De Jonge, R. & Pieterse, C. M. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 60, 1405–1419 (2019).
Su, P. et al. Microbiome homeostasis on rice leaves is regulated by a precursor molecule of lignin biosynthesis. Nat. Commun. 15, 23 (2024).
Xu, P. et al. Temporal metabolite responsiveness of microbiota in the tea plant phyllosphere promotes continuous suppression of fungal pathogens. J. Adv. Res. 39, 49–60 (2022).
He, X. et al. Heritable microbiome variation is correlated with source environment in locally adapted maize varieties. Nat. Plants 10, 598–617 (2024).
Wintermans, P. C., Bakker, P. A. & Pieterse, C. M. Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol. Biol. 90, 623–634 (2016).
Beckers, B. et al. Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc. Natl Acad. Sci. USA 113, 2312–2317 (2016).
Stringlis, I. A. et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl Acad. Sci.USA 115, E5213–E5222 (2018).
Escudero-Martinez, C. et al. Identifying plant genes shaping microbiota composition in the barley rhizosphere. Nat. Commun. 13, 3443 (2022).
Oyserman, B. O. et al. Disentangling the genetic basis of rhizosphere microbiome assembly in tomato. Nat. Commun. 13, 3228 (2022).
Escudero-Martinez, C. & Bulgarelli, D. Engineering the crop microbiota through host genetics. Annu. Rev. Phytopathol. 61, 257–277 (2023).
Sasse, J., Martinoia, E. & Northen, T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23, 25–41 (2018).
Njaci, I. et al. Chromosome-level genome assembly and population genomic resource to accelerate orphan crop lablab breeding. Nat. Commun. 14, 1915 (2023).
Shorinola, O. et al. Integrative and inclusive genomics to promote the use of underutilised crops. Nat. Commun. 15, 320 (2024).
Acknowledgements
The help of Martin Stonitsch (Graz, Austria) in preparing figure elements with the assistance of AI-generated rendering by Midjourney and Adobe Firefly is highly appreciated. The author also wants to thank Mark Chapman (University of Southampton) for discussions related to underutilized crops.
Author information
Authors and Affiliations
Contributions
T.C. conceptualized and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Communications thanks Jos Raaijmakers, Joelle Sasse and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cernava, T. Coming of age for Microbiome gene breeding in plants. Nat Commun 15, 6623 (2024). https://doi.org/10.1038/s41467-024-50700-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50700-7
This article is cited by
-
Amino-acid-transporter-mediated assembly of rhizosphere microbiota enhances soil organic nitrogen acquisition in rice
Nature Plants (2026)
-
Developing Effective Bioinoculant and Engineering Plant Microbiome for Climate Resilient Agriculture: Lessons Learned and Future Roadmap
Current Microbiology (2026)
-
Unveiling essential host genes and keystone microorganisms of the olive tree holobiont linked to Verticillium wilt tolerance
Microbiome (2025)
-
Reconsidering the ecological effect of seed endophytes in building plant microbial environments: lessons from a Chinese medicinal plant Panax notoginseng
Environmental Microbiome (2025)
-
Host metabolites explain microbiome variation between different rice genotypes
Microbiome (2025)