Abstract
In plants, the conserved plant-specific photoreceptor UV RESISTANCE LOCUS 8 (UVR8) perceives ultraviolet-B (UV-B) light and mediates UV-B-induced photomorphogenesis and stress acclimation. In this study, we reveal that UV-B light treatment shortens seedlings, increases stem thickness, and enhances UV-B stress tolerance in rice (Oryza sativa) via its two UV-B photoreceptors OsUVR8a and OsUVR8b. Although the rice and Arabidopsis (Arabidopsis thaliana) UVR8 (AtUVR8) photoreceptors all form monomers in response to UV-B light, OsUVR8a, and OsUVR8b function is only partially conserved with respect to AtUVR8 in UV-B-induced photomorphogenesis and stress acclimation. UV-B light and CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) promote the nuclear accumulation of AtUVR8; by contrast, OsUVR8a and OsUVR8b constitutively localize to the nucleus via their own nuclear localization signals, independently of UV-B light and the RING-finger mutation of OsCOP1. We show that OsCOP1 negatively regulates UV-B responses, and shows weak interaction with OsUVR8s, which is ascribed to the N terminus of OsCOP1, which is conserved in several monocots. Furthermore, transcriptome analysis demonstrates that UV-B-responsive gene expression differs globally between Arabidopsis and rice, illuminating the evolutionary divergence of UV-B light signaling pathways between monocot and dicot plants.
Similar content being viewed by others
Introduction
To acclimate to constantly changing light conditions, plants have multiple photoreceptor-dependent light-sensing systems that regulate diverse aspects of their growth and development. Non-damaging ultraviolet-B (UV-B) light acts as an informational signal that mediates seedling photomorphogenesis, organ development, floral transition, and stress acclimation1. UV-B light is perceived by UV RESISTANCE LOCUS 8 (UVR8), a highly conserved plant-specific UV-B photoreceptor2. Originally identified in Arabidopsis (Arabidopsis thaliana), UVR8 comprises a seven-bladed β-propeller in which some of its tryptophan residues function as UV-B-absorbing chromophores. Upon UV-B exposure, UVR8 is activated to rapidly change its quaternary structure from a homodimer to a monomer2,3,4 and accumulates in the nucleus, where it mediates UV-B light signal transduction5,6,7.
The E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) is a crucial signaling factor in light regulation of plant development. In darkness, COP1 associates with SUPPRESSOR OF PHYA-105 proteins (SPA1–SPA4), and the COP1–SPA core complexes function as substrate receptors of a CULLIN 4 (CUL4)–DAMAGED DNA BINDING PROTEIN 1 (DDB1)-based E3 ligase complex, repressing photomorphogenesis by mediating the ubiquitination and degradation of photomorphogenesis-promoting transcription factors such as ELONGATED HYPOCOTYL 5 (HY5)8. Under light conditions, cryptochrome photoreceptors and UVR8 outcompete HY5 for binding to COP1, thus stabilizing HY5 and initiating the light-responsive transcriptome program9,10.
Specifically, in response to UV-B light, monomerized UVR8 interacts with the WD40 domain of COP1 via its core domain and C-terminal Val‐Pro (VP) motif, which releases COP1–SPAs from CUL4–DDB111,12,13,14. Following this interaction, COP1 promotes UVR8 nuclear retention, allowing UVR8 to accumulate and activate its downstream signaling cascade in the nucleus15. The two-interface interaction between UVR8 and COP1 can be disturbed by two WD40 proteins whose encoding genes are UV-B-inducible, REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and its homolog RUP213,16,17. RUP1 and RUP2 are recruited into the CUL4–DDB1 E3 ligase as substrate receptors to mediate HY5 degradation, while they are destabilized by COP1 to ultimately stabilize HY5 under UV-B light18. UVR8 reverts to its ground state via dimerization facilitated by RUP1 and RUP219,20. Recent structural studies have pinpointed how RUP2 binds to UVR8 through a similar two-interface interaction mode with UVR8–COP1WD40 to regulate the UVR8 photocycle14,21.
UVR8 and its signaling pathway originated in chlorophytes during evolution and conferred green plants with the ability to cope with UV-B radiation22,23. The functional conservation of UVR8 has been explored in various members of the green lineage, such as the unicellular alga Chlamydomonas reinhardtii, the liverwort Marchantia polymorph, and the dicot plant tomato (Solanum lycopersicum)24,25,26,27,28. The molecular mechanisms by which UVR8 signaling governs plant development have been elucidated primarily using Arabidopsis as a model dicot. However, how UV-B light and UVR8 regulate crop growth in monocots is less understood. In rice (Oryza sativa), low-level UV-B light (10−1 to 10−3 μmol m−2 s−1) inhibits coleoptile growth and enhances abiotic stress tolerance29. The rice genome contains two UVR8 homologous genes, OsUVR8a (Os02g0554100) and OsUVR8b (Os04g0435700). Their predicted protein products are 82% identical to each other and 74% and 75% identical, respectively, to Arabidopsis UVR829. However, the molecular mechanism of UV-B light signal transduction in rice remains largely unknown.
In this study, we explored how UV-B light signaling regulates rice seedling development and stress acclimation. We confirmed OsUVR8a and OsUVR8b as rice UV-B photoreceptors and identified OsCOP1 as a negative regulator of UV-B signaling. We demonstrated that OsUVR8a and OsUVR8b exhibit a similar conformational response and partial functional conservation to Arabidopsis UVR8, but display a distinct subcellular localization and protein–protein interaction mode shared in several other monocots, suggestive of evolutionary divergence of UV-B light signaling between monocot and dicot plants.
Results
OsUVR8a and OsUVR8b are required for UV-B regulation of rice development and acclimation
To study how rice seedlings physiologically respond to UV-B light signals, we designed an experimental system to analyze the effects of non-damaging UV-B light on rice seedling development. We grew rice seedlings under short-day conditions (10-h light/14-h dark photoperiod) in white light alone (WL) or WL supplemented with 15 μW cm−2 (equivalent to ~2.25 μmol m−2 s−1) narrowband UV-B light (WL + UV-B) during the daytime. Under these conditions, 10-day-old seedlings for the two wild-type (WT) cultivars, Dongjin (DJ), and Nipponbare (Nip), were shorter in stature, with shorter first and second leaf sheaths, when grown under WL + UV-B compared to WL (Supplementary Fig. 1a–c). At the same time, stem thickness increased under WL + UV-B relative to WL (Supplementary Fig. 1a, d, e). These results demonstrate that low-level UV-B light promotes the development of a compact seedling architecture in rice.
To examine the contribution of putative UV-B photoreceptors responsible for rice seedling development, we compared the protein sequences among AtUVR8, OsUVR8a, and OsUVR8b. The tryptophan residues and GWRHT and VP motifs, which are critical for AtUVR8 photoreceptor activity and signal transduction1, are well conserved in OsUVR8a and OsUVR8b. However, we noticed lower conservation in the N termini of OsUVR8s, with an unclear function, and their C termini, which include the C27 domain that is pivotal for AtUVR8 interaction with signaling factors (Fig. 1a and Supplementary Fig. 1f). Moreover, structural modeling based on the reported AtUVR8 core structure demonstrated that OsUVR8a, OsUVR8b and AtUVR8 have identical overall structures, forming a seven-bladed β-propeller. The extensive network of intramolecular interactions was highly conserved, consisting of dimer-stabilizing arginines and UV-B-perceiving tryptophans (Fig. 1b). Thus, OsUVR8a and OsUVR8b share sequence and structural similarity with AtUVR8.
a Domain structures of AtUVR8, OsUVR8a, and OsUVR8b. The positions of tryptophan residues (asterisks), GWRHT domains, and the VP motif are indicated. b AtUVR8 structure and predicted structural models of OsUVR8a and OsUVR8b, with GWRHT domains highlighted in insets. c Representative images of 10-day-old seedlings from wild type (DJ) and the Osuvr8a Osuvr8b (Osuvr8ab) double mutants Osuvr8ab 3-1 and Osuvr8ab 5-4 grown under WL only (200 μmol m−2 s−1) or WL supplemented with UV-B (WL + UV-B; UV-B: 15 μW cm−2; equivalent to ~2.25 μmol m−2 s−1). Scale bar, 5 cm. The diagrams below the images indicate the mutation in Osuvr8ab 3-1 and Osuvr8ab 5-4. d Height of the seedlings in c. Data are means ± standard deviation (SD). Different lowercase letters indicate statistically significant differences by one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test (P < 0.05). e Representative images of stem cross sections from 10-day-old WT DJ and Osuvr8ab seedlings grown under WL or WL + UV-B. Scale bar, 5 mm. f Cross-sectional area of the stems in e. Data are means ± SD; n = 15. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). g Representative images of the second leaf from WT DJ and Osuvr8ab seedlings grown under non-acclimated or acclimated conditions before UV-B stress treatment. h qRT-PCR analysis of the relative transcript levels of OsCHS1, OsLAR, and OsCHI in WT DJ and Osuvr8ab seedlings grown under WL alone or WL + UV-B. qRT-PCR data were normalized to UBQ, with the transcript levels in WT DJ under WL set to 1. Data are means ± SD; n = 3 biological replicates. Source data of (d, f, h) are provided as a Source Data file.
We generated Osuvr8a Osuvr8b (Osuvr8ab) double mutants by clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease 9 (CRISPR/Cas9)-mediated gene editing to abolish OsUVR8a and OsUVR8b function in the DJ background. We obtained two independent mutant lines with frameshift and nonsense mutations in both genes (Supplementary Fig. 1g). Unlike the WT DJ, Osuvr8ab mutants did not show changes in response to UV-B light in seedling height, lengths of the first and second leaf sheaths, or stem thickness (Fig. 1c–f and Supplementary Fig. 1h). These results demonstrate that OsUVR8a and OsUVR8b are required for UV-B-regulated seedling development in rice. In Arabidopsis, seedlings pretreated with low-level UV-B light establish a UV-B acclimation response and accumulate sunscreen flavonoids, resulting in enhanced tolerance of UV-B stress, in a UVR8-dependent manner11. To test whether UV-B light promotes a similar stress acclimation response in rice, we treated 10-day-old rice seedlings grown under WL (non-acclimated) or WL + UV-B (acclimated) conditions with 250 μW cm−2 (equivalent to ~37.50 μmol m−2 s−1) broadband UV-B light as stress for 2 days, followed by recovery in WL for 1 day. Non-acclimated WT DJ seedlings were hypersensitive to UV-B stress, as evidenced by their brown and shriveled leaves, whereas acclimated seedlings were more stress tolerant (Fig. 1g). However, Osuvr8ab mutants were hypersensitive to UV-B stress regardless of prior acclimation (Fig. 1g). Consistent with these phenotypes, the expression of three flavonoid biosynthetic genes, CHALCONE SYNTHASE 1 (OsCHS1), LEUCOANTHOCYANIDIN REDUCTASE (OsLAR) and CHALCONE-FLAVONONE ISOMERASE (OsCHI), was notably induced by UV-B pretreatment in WT DJ, but not in the Osuvr8ab mutants (Fig. 1h). These results suggest that UV-B acclimation promotes UV-B stress tolerance via OsUVR8a and OsUVR8b in rice.
We also generated transgenic rice lines harboring transgenes encoding OsUVR8a or OsUVR8b fused to CYAN FLUORESCENT PROTEIN (CFP) and driven by the cauliflower mosaic virus (CaMV) 35S promoter in the Nip background; we characterized two independent lines for each construct (Supplementary Fig. 2a). The CFP-OsUVR8a overexpression lines displayed enhanced UV-B-mediated inhibition of seedling height and leaf sheath length, as well as thicker stems than WT Nip (Fig. 2a–d and Supplementary Fig. 2b). Similarly, the CFP-OsUVR8b overexpression lines showed decreased height and leaf sheath length, along with increased stem thickness, even without supplemental UV-B irradiation (Fig. 2a–d and Supplementary Fig. 2b). After UV-B acclimation, CFP-OsUVR8a lines showed slightly elevated stress tolerance, while CFP-OsUVR8b lines had markedly elevated stress tolerance and expression of flavonoid biosynthetic genes, compared to WT Nip (Fig. 2e–h). To confirm the role of OsUVR8a and OsUVR8b in these responses, we generated transgenic rice lines constitutively expressing CFP-OsUVR8a or CFP-OsUVR8b in the Osuvr8ab mutant background (Supplementary Fig. 2c). We found that both CFP-OsUVR8a/Osuvr8ab and CFP-OsUVR8a/Osuvr8ab rescued Osuvr8ab and responded well to UV-B light by showing reduced plant height, increased stem thickness, and induced gene expression (Supplementary Fig. 2d–i). Collectively, these results demonstrate that OsUVR8a and OsUVR8b positively regulate UV-B-mediated seedling development and acclimation.
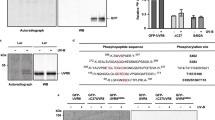
a Representative images of 10-day-old WT Nip, CFP-OsUVR8a and CFP-OsUVR8b seedlings grown under WL or WL + UV-B. Scale bar, 5 cm. b Height of the seedlings in a. Data are means ± SD. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). c Representative images of stem cross sections from 10-day-old WT Nip, CFP-OsUVR8a, and CFP-OsUVR8b seedlings grown under WL or WL + UV-B. Scale bar, 5 mm. d Cross-sectional area of the stems in c. Data are means ± SD; n = 15. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). e Representative images of the second leaf from WT Nip, CFP-OsUVR8a and CFP-OsUVR8b seedlings grown under non-acclimated or acclimated conditions before UV-B stress (250 μW cm−2; equivalent to ~37.50 μmol m−2 s−1). Scale bar, 2 mm. f–h qRT-PCR analysis of the relative transcript levels of OsCHS1 (f), OsLAR (g), and OsCHI (h) in WT Nip, CFP-OsUVR8a and CFP-OsUVR8b seedlings grown under WL or WL + UV-B. Transcript levels were normalized to UBQ, with the transcript levels in WT Nip under WL set to 1. Data are means ± SD; n = 3 biological replicates. i Conformation of CFP-OsUVR8a and CFP-OsUVR8b in rice seedlings grown under WL or WL + UV-B. Total proteins were assayed by SDS-PAGE without heat denaturation followed by immunoblot analysis using anti-GFP antibodies. d dimer, m monomer. Anti-PHOSPHOENOLPYRUVATE CARBOXYLASE (PEPC) was used as a loading control. Three independent experiments were performed and one representative result is presented. j Confocal micrographs of CFP-OsUVR8a and CFP-OsUVR8b in leaf sheath cells from 10-day-old CFP-OsUVR8a and CFP-OsUVR8b seedlings grown under WL or WL + UV-B. Scale bars, 50 μm. Source data of (b, d, f–i) are provided as a Source Data file.
Rice and Arabidopsis UVR8 orthologs show a mixture of functional conservation and divergence
To test the functional conservation of OsUVR8a and OsUVR8b with AtUVR8, we examined the UV-B responsiveness of OsUVR8a and OsUVR8b at the protein and subcellular levels. We checked the UV-B-induced conformational change of OsUVR8s, as previously established in Arabidopsis2, using rice protein extracts separated by SDS-PAGE but without prior heat denaturation. Without UV-B irradiation, we detected dimeric and monomeric CFP-OsUVR8a and OsUVR8b. Following UV-B irradiation, both proteins mostly accumulated as monomers (Fig. 2i). This result suggests that like AtUVR8, OsUVR8a and OsUVR8b perceive UV-B light and undergo a dimer-to-monomer switch, consistent with the high conservation between the structures of OsUVR8a and OsUVR8b and of AtUVR8 (Fig. 1b). In sharp contrast to AtUVR8, which shows cytoplasmic and nuclear localization with greater nuclear accumulation under UV-B light5,7, OsUVR8a and OsUVR8b displayed constitutive nuclear localization with no clear effect from UV-B light treatment in rice leaf sheath cells (Fig. 2j), and in rice protoplasts co-transfected with CFP-OsUVR8a or CFP-OsUVR8b and mCherry-H2B (encoding a fusion between the red fluorescent protein mCherry and histone H2B) as a nuclear indicator (Supplementary Fig. 3). These data suggest a distinct localization mechanism for OsUVR8a and OsUVR8b from that of AtUVR8.
We next asked whether OsUVR8s would functionally replace AtUVR8 by generating transgenic Arabidopsis lines overexpressing CFP-OsUVR8a or CFP-OsUVR8b from the 35 S promoter in the uvr8-6 null mutant background. We characterized two independent lines per construct, with CFP-OsUVR8 abundance comparable to that of YFP-AtUVR8/uvr8-6 (Supplementary Fig. 4a). In Arabidopsis, low-level UV-B light inhibits seedling hypocotyl elongation during photomorphogenic development and mediates UV-B stress acclimation in a UVR8-dependent manner11. We observed an inhibition of hypocotyl elongation by UV-B light in CFP-OsUVR8a/uvr8-6 and CFP-OsUVR8b/uvr8-6, although this inhibition was weaker than that in YFP-AtUVR8/uvr8-6 seedlings (Fig. 3a, b). To test the acclimatory response of these transgenic lines to UV-B light, we examined their growth recovery after irradiation with UV-B stress following or without prior low-level UV-B treatment. The acclimated CFP-OsUVR8a/uvr8-6 and CFP-OsUVR8b/uvr8-6 seedlings showed stronger tolerance against UV-B stress than uvr8-6, but remained more sensitive than Col-0 and YFP-AtUVR8/uvr8-6 (Supplementary Fig. 4b). In agreement with this result, the survival rates of CFP-OsUVR8a/uvr8-6 and CFP-OsUVR8b/uvr8-6 seedlings were higher than that of uvr8-6 but lower than those of Col-0 and YFP-AtUVR8/uvr8-6 (Supplementary Fig. 4c). The expression of three typical UV-B-inducible marker genes, EARLY LIGHT-INDUCIBLE PROTEIN 1 (ELIP1), ELIP2 and CHI, was induced by UV-B light in CFP-OsUVR8a/uvr8-6 and CFP-OsUVR8b/uvr8-6. However, the expression of ELIP1 and ELIP2 in CFP-OsUVR8a/uvr8-6 was induced to a lesser degree than in YFP-AtUVR8/uvr8-6, while the expression of all three genes in CFP-OsUVR8b/uvr8-6 was induced to a lesser degree than in YFP-AtUVR8/uvr8-6 and CFP-OsUVR8a/uvr8-6 (Fig. 3c–e). These results demonstrate that OsUVR8a and OsUVR8b do not fully rescue uvr8-6 to the extent that AtUVR8 does, suggesting partial functional conservation between OsUVR8s and AtUVR8 in UV-B-induced photomorphogenesis and stress acclimation.
a Representative images of 4-day-old WT (Col-0), uvr8-6, YFP-AtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, and CFP-OsUVR8b/uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Scale bar, 1 mm. b Hypocotyl length of the seedlings in a. Data are means ± SD; n = 25. Percentages listed above bars indicate relative hypocotyl length under +UV-B normalized to that under −UV-B for each line. c–e qRT-PCR analysis of the relative transcript levels of ELIP1 (c), ELIP2 (d), and CHI (e) in Col-0, uvr8-6, YFP-AtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, and CFP-OsUVR8b/uvr8-6 seedlings grown under −UV-B or +UV-B. Transcript levels were normalized to ACTIN, with the transcript levels in Col-0 under −UV-B set to 1. Data are means ± SD; n = 3 biological replicates. f Quantitative yeast two-hybrid (Y2H) assays showing the interaction between UVR8 and AtCOP1 in yeast cultures exposed to −UV-B or +UV-B conditions. AD B42 activation domain, BD LexA binding domain, Empty empty vector. Data are means ± SD; n = 5 biological replicates. g Luciferase complementation imaging (LCI) assays showing the interaction between UVR8 and AtCOP1 in N. benthamiana. N. benthamiana leaves were co-infiltrated with AtUVR8-nLuc, OsUVR8a-nLuc, OsUVR8b-nLuc or nLuc, and cLuc-AtCOP1 or cLuc as indicated. h Co-immunoprecipitation (Co-IP) assay with anti-GFP antibodies using 4-day-old YFP-AtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, and CFP-OsUVR8b/uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Immunoblot analysis was performed with anti-GFP and anti-COP1 antibodies. Anti-RPN6 was used as a negative control. i, j Nuclear fractionation assay of the subcellular distribution of YFP-AtUVR8 in 4-day-old YFP-AtUVR8/uvr8-6 Arabidopsis seedlings grown under −UV-B or +UV-B conditions (i), of CFP-OsUVR8a and CFP-OsUVR8b in 10-day-old CFP-OsUVR8a and CFP-OsUVR8b rice seedlings grown under WL or WL + UV-B (j). Anti-PEPC and anti-histone H3 were used as cytosolic and nuclear markers, respectively. Source data of (b–f, h–j) are provided as a Source Data file.
The ability of AtUVR8 to mediate UV-B signaling relies on its monomerization and subsequent interaction with COP1 in response to UV-B light11. Considering the partial functional conservation of OsUVR8a and OsUVR8b with AtUVR8 in rescuing uvr8-6 (Fig. 3a–e and Supplementary Fig. 4b, c), we investigated whether this difference stemmed from differences in biochemical properties. We thus tested their dimer-to-monomer switch induced by UV-B light in transgenic Arabidopsis seedlings. Similarly to AtUVR8, most CFP-OsUVR8a and CFP-OsUVR8b formed dimers under −UV-B conditions and converted to their monomeric form under +UV-B conditions (Supplementary Fig. 4d). We then examined the interaction of OsUVR8a and OsUVR8b with AtCOP1. In yeast two-hybrid (Y2H) assays, AtCOP1 strongly interacted with AtUVR8 under +UV-B, and interacted to a weaker extent with OsUVR8a or OsUVR8b (Fig. 3f). In firefly luciferase (Luc) complementation imaging (LCI) assays conducted in Nicotiana benthamiana leaves, we readily detected a UV-B-induced interaction between AtCOP1 and OsUVR8a or OsUVR8b, but with an overall weaker interaction strength than that between AtCOP1 and AtUVR8 (Fig. 3g). Moreover, co-immunoprecipitation (Co-IP) assays with anti-GFP antibodies showed that AtCOP1 is co-immunoprecipitated with CFP-OsUVR8a and CFP-OsUVR8b specifically under +UV-B, but the amount of co-immunoprecipitated AtCOP1 was lower than with YFP-AtUVR8 (Fig. 3h). Taken together, these results suggest that OsUVR8a and OsUVR8b possess a lower affinity for AtCOP1 than AtUVR8, consistent with the incomplete activity of OsUVR8a and OsUVR8b in UV-B-induced photomorphogenesis and stress acclimation in transgenic Arabidopsis seedlings.
In Arabidopsis, UVR8 exhibits both cytoplasmic and nuclear localization in the absence of UV-B light, while UV-B irradiation facilitates its nuclear accumulation. This nuclear accumulation is necessary for activated UVR8 to mediate UV-B signaling and is positively regulated by COP15,6,7. To investigate whether OsUVR8s are subject to similar regulation, we employed confocal microscopy to compare their subcellular localization patterns in response to UV-B light. When their encoding constructs were expressed in N. benthamiana leaves, we detected CFP-OsUVR8a and CFP-OsUVR8b in the nucleus regardless of UV-B light treatment (Supplementary Fig. 4e). We also performed a nuclear fractionation assay using transgenic Arabidopsis and rice seedlings, separating total proteins into cytosolic and nuclear fractions. This indicated that UV-B light elevates the nuclear abundance of AtUVR8 while not affecting that of OsUVR8a or OsUVR8b (Fig. 3i, j). Together with the constitutive nuclear localization of the two proteins in rice (Fig. 2j and Supplementary Fig. 3), these data lead us to conclude that OsUVR8a and OsUVR8b are subject to a regulatory mechanism distinct from that of AtUVR8.
To gain molecular insight into the regulation of OsUVR8a and OsUVR8b subcellular localization, we looked for a putative nuclear localization signal (NLS). Indeed, we identified a putative NLS in AtUVR8, OsUVR8a, and OsUVR8b (Fig. 4a). To test whether these putative NLSs are functional, we generated transgenic Arabidopsis lines expressing YFP-mAtUVR8, CFP-mOsUVR8a, or CFP-mOsUVR8b in the uvr8-6 background encoding UVR8 variants with a mutated NLS. Although YFP-mAtUVR8 was less abundant than YFP-AtUVR8 (Supplementary Fig. 4f), YFP-mAtUVR8 expression rescued uvr8-6, as indicated by a comparable UV-B-induced inhibition of hypocotyl elongation to that seen in Col-0 (Fig. 4b and Supplementary Fig. 4g). However, the inhibition was weaker in CFP-mOsUVR8a/uvr8-6 and CFP-mOsUVR8b/uvr8-6 than that in CFP-OsUVR8a/uvr8-6 and CFP-OsUVR8b/uvr8-6, respectively (Fig. 4b and Supplementary Fig. 4g). Consistent with the above phenotypes, the expression of ELIP2, CHS and F3’H was markedly induced by UV-B light in YFP-mAtUVR8/uvr8-6 and Col-0 (Fig. 4, c–e). However, the induction was less strongly induced in CFP-mOsUVR8a/uvr8-6 and CFP-mOsUVR8b/uvr8-6 relative to their intact counterparts (Fig. 4c–e).
a Multiple protein sequence alignment showing the mutation sites (red) in the putative NLS of AtUVR8, OsUVR8a, and OsUVR8b. b Hypocotyl length of seedlings from the indicated genotypes in Supplementary Fig. 4g. Data are means ± SD. Percentages above bars indicate relative hypocotyl length under +UV-B condition relative to that under −UV-B for each line. c–e qRT-PCR analysis of the relative transcript levels of ELIP2 (c), CHS (d), and F3’H (e) in Col-0, uvr8-6, YFP-mAtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, CFP-mOsUVR8a/uvr8-6, CFP-OsUVR8b/uvr8-6, and CFP-mOsUVR8b/uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Transcript levels were normalized to ACTIN, with the transcript levels in Col-0 or CFP-OsUVR8a/uvr8-6 under −UV-B set to 1. Data are means ± SD; n = 3 biological replicates. f Confocal micrographs showing the subcellular localization of YFP-AtUVR8, YFP-mAtUVR8, CFP-OsUVR8a, CFP-mOsUVR8a, CFP-OsUVR8b, and CFP-mOsUVR8b in YFP-AtUVR8/uvr8-6, YFP-mAtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, CFP-mOsUVR8a/uvr8-6, CFP-OsUVR8b/uvr8-6, and CFP-mOsUVR8b/uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Nuclei were counterstained with DAPI. Yellow, YFP signal; green, CFP signal; blue, DAPI signal. Scale bars, 50 μm. Source data of (b–e) are provided as a Source Data file.
Furthermore, confocal microscopy showed that all three proteins with a mutated NLS accumulate in the cytoplasm in the absence of UV-B light, confirming that each NLS in AtUVR8, OsUVR8a, and OsUVR8b is functional (Fig. 4f). When exposed to UV-B light, YFP-mAtUVR8 still translocated to the nucleus, like its intact counterpart. By contrast, CFP-mOsUVR8a and CFP-mOsUVR8b remained in the cytoplasm (Fig. 4f). Moreover, although the cop1-4 mutation compromised the enhanced nuclear accumulation of AtUVR8 under +UV-B conditions, as previously reported6,7, it had little effect on the nuclear localization of OsUVR8a and OsUVR8b in transgenic Arabidopsis lines (Supplementary Fig. 4h). Collectively, these results demonstrate that in contrast to AtUVR8, the nuclear localization of OsUVR8a and OsUVR8b relies on their own NLS but is independent of UV-B light, and the regulation of AtUVR8 subcellular partitioning requires alternative regulators beside its NLS, such as COP1.
OsCOP1 and AtCOP1 are functionally divergent in UV-B-mediated seedling development
Considering the distinct modes of protein interaction and subcellular localization between UVR8 orthologs in Arabidopsis and rice, we wondered whether COP1 orthologs might play different roles in UVR8 signaling in these two species. OsCOP1 (encoded by Os02g0771100) was previously identified as an ortholog of AtCOP1 in rice30,31. Protein sequence alignment confirmed that OsCOP1 is 70.2% identical to AtCOP1 and contains a Zn-binding RING-finger domain, coiled-coil region, bipartite NLS, and WD40-repeat domain (Supplementary Fig. 5a). To investigate whether OsCOP1 is involved in UV-B-regulated seedling architecture and stress acclimation, we employed CRISPR/Cas9-mediated gene editing to obtain Oscop1 mutants. Line 3-1 in the DJ background harbors a 3-bp deletion removing cysteine 96 (C96) within the Zn-binding RING finger of OsCOP1 (Supplementary Fig. 5b), a conserved residue for COP1 E3 ligase activity32; this mutant developed shorter seedlings than WT DJ in both WL and dark conditions (Supplementary Fig. 5c, d). Line 2 in the Nip background carries frameshift and nonsense mutations within the Zn-binding RING-finger domain that lead to embryonic lethality in the homozygous state, prompting us to use heterozygous seedlings for phenotypic analysis (Supplementary Fig. 5e).
COP1 is required for UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. The cop1-4 mutant develops short hypocotyls that are shortened slightly further by UV-B light exposure, and does not tolerate UV-B stress. This mutant is defective in UV-B-responsive transcriptome changes11,33 and has disrupted nuclear retention of UVR87,15. In Oscop1 Line 3-1 and Oscop1/OsCOP1 Line 2 seedlings, however, UV-B light effectively decreased seedling height and increased UV-B stress acclimation (Fig. 5a, b and Supplementary Fig. 5e–g). The expression of flavonoid biosynthetic genes was also induced, and to a comparable or greater extent in Oscop1 mutants than in the WT DJ (Fig. 5c–f). Then we performed nuclear fractionation assays to examine the protein levels of endogenous OsUVR8s in total and nuclear fractions of WT DJ and Oscop1 mutants. In both genotypes, the abundance of OsUVR8s in the total and nuclear fractions was comparable and not regulated by UV-B light (Fig. 5g). Moreover, we prepared protoplasts from the Oscop1 mutants (Line 3-1) to analyze the effect of this mutation on the nuclear localization of OsUVR8s. Using mCherry-H2B as a nuclear marker reporter in Oscop1 protoplasts, CFP-OsUVR8a and CFP-OsUVR8b still localized to the nucleus, and this was not influenced by UV-B light (Supplementary Fig. 5h). These results demonstrate that the nuclear accumulation of OsUVR8s is independent of UV-B light and the RING-finger mutation of OsCOP1, suggesting the differentiation of UVR8 signaling pathways between Arabidopsis and rice.
a Representative images of WT DJ and Oscop1 seedlings grown under WL or WL + UV-B. Scale bar, 5 cm. The diagrams below the images indicate the mutation in Oscop1 3-1. b Height of the seedlings in a. Data are means ± SD. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). c–f qRT-PCR analysis of the relative transcript levels of OsCHS1 (c), OsC1-Myb (d), OsLAR (e), and OsCHS28 (f) in WT DJ and Oscop1 seedlings grown under WL or WL + UV-B. Transcript levels were normalized to UBQ, with transcript levels in WT DJ under WL set to 1. Data are means ± SD; n = 3 biological replicates. Numbers above the bars indicate fold induction. g Nuclear fractionation assay of the nuclear accumulation of OsUVR8s in 10-day-old WT DJ and Oscop1 rice seedlings grown under WL or WL + UV-B. Histone H3 was used as a loading control and a nuclear marker. h Representative images of 4-day-old Col-0, cop1-4, YFP-AtCOP1/cop1-4, and CFP-OsCOP1/cop1-4 seedlings grown under −UV-B or +UV-B. Scale bar, 1 mm. i Hypocotyl length of seedlings in h. Data are means ± SD; n = 20. For each line, the percentage indicates the relative hypocotyl length under +UV-B normalized to that under −UV-B. j Quantitative Y2H assays showing the interaction between AtUVR8 and COP1 in yeast cultures exposed to −UV-B or +UV-B conditions. AD B42 activation domain, BD LexA binding domain, Empty empty vector. Data are means ± SD; n = 5 biological replicates. k LCI assays showing the interaction between AtUVR8 and COP1 in N. benthamiana. N. benthamiana leaves were co-infiltrated with AtUVR8-nLuc or nLuc, and cLuc-AtCOP1, cLuc-OsCOP1, or cLuc as indicated. l Co-IP assays with anti-GFP antibodies using 4-day-old YFP-AtCOP1/cop1-4 and CFP-OsCOP1/cop1-4 seedlings grown under −UV-B or +UV-B conditions. Immunoblot analysis was performed with anti-GFP and anti-UVR8 antibodies. Anti-RPN6 was used as a loading and non-immunoprecipitated control. Source data of (b–g, i, j, l) are provided as a Source Data file.
To investigate the mechanism behind the functional conservation and divergence between OsCOP1 and AtCOP1, we generated Arabidopsis transgenic lines expressing CFP-OsCOP1 driven by the 35 S promoter in the cop1-4 mutant background (Supplementary Fig. 6a). In both darkness and WL conditions, overexpression of CFP-OsCOP1 fully rescued cop1-4, resulting in normal skotomorphogenic and photomorphogenic development, respectively (Supplementary Fig. 6b). By contrast, CFP-OsCOP1 overexpression did not rescue cop1-4, as CFP-OsCOP1/cop1-4 seedlings showed an impaired response to UV-B light in terms of inhibition of hypocotyl elongation and the induction of UV-B-responsive gene expression (Fig. 5h, i and Supplementary Fig. 6c–e). Consistent with the observed UV-B response in Oscop1 mutants, these results suggest that OsCOP1 does not have the same functions as AtCOP1, specifically in the regulation of UV-B-induced photomorphogenesis. We then sought to determine whether OsCOP1 regulates UV-B signal transduction in these transgenic Arabidopsis seedlings by analyzing the formation of the UVR8–COP1 complex, the pivotal UV-B signaling module. Compared to the strong UV-B-induced interaction of AtCOP1 with AtUVR8, OsCOP1 displayed a rather low affinity for AtUVR8 in Y2H, LCI, and Co-IP assays (Fig. 5j–l). These results further support a functional divergence between OsCOP1 and AtCOP1 in UV-B-mediated seedling development.
Arabidopsis and rice UVR8–COP1 have distinct interaction modes
As OsCOP1 and AtCOP1 differentially mediated UV-B signal transduction, we asked whether these differences could be ascribed to specific functional domains in these proteins. We examined the direct interaction of OsUVR8s with full-length OsCOP1 in LCI assays, which revealed a relatively weak but UV-B-inducible interaction (Fig. 6a). However, Y2H assays showed no direct interaction (Supplementary Fig. 7a), suggesting very weak or transient binding of full-length OsCOP1 to OsUVR8s.
a LCI assays showing the interaction of OsUVR8a and OsUVR8b with OsCOP1 in N. benthamiana leaves co-infiltrated with OsUVR8a-nLuc, OsUVR8b-nLuc, or nLuc, and cLuc-OsCOP1 or cLuc as indicated. The AtUVR8-nLuc and cLuc-AtCOP1 pair were used as a positive control. b Quantitative Y2H assays showing the interaction of AtUVR8 with AtCOP1C or OsCOP1C in yeast exposed to −UV-B or +UV-B conditions. Data are means ± SD; n = 5 biological replicates. c Diagram of the COP1 variants used in d, e. The AtCOP1 and OsCOP1 fragments are presented in blue and dark orange, respectively. d LCI assays showing the interaction of AtUVR8 with intact and chimeric COP1 proteins in N. benthamiana leaves co-infiltrated with AtUVR8-nLuc or nLuc, and cLuc-AtCOP1, cLuc-Swap_At1, cLuc-OsCOP1, cLuc-Swap_Os or cLuc as indicated. e Quantitative Y2H assays showing the interaction of AtUVR8 with intact and chimeric COP1 proteins. Data are means ± SD; n = 5 biological replicates. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). f Phylogram of COP1 homologs. COP1 homologs in monocots and dicots are shown in red and blue, respectively. At A. thaliana, Md Malus domestic, Gm Glycine max, Sl S. lycopersicum, Os O. sativa, Ta Triticum aestivum, Zm Zea mays, Sb S. bicolor, Si Setaria italica, Pm Panicum miliaceum, Hv Hordeum vulgare. g LCI assays showing the interaction of AtUVR8 and HvCOP1 or SbCOP1 in N. benthamiana leaves co-infiltrated with AtUVR8-nLuc or nLuc, and cLuc-HvCOP1, cLuc-SbCOP1, or cLuc as indicated. The AtUVR8-nLuc and cLuc-AtCOP1 pair were used as a positive control. h Diagram of the COP1 variants used in (i, j). The AtCOP1, HvCOP1, and SbCOP1 fragments are presented in blue, pink and light orange, respectively. i, j Quantitative Y2H assays showing the interaction of AtUVR8 with intact and chimeric COP1 proteins. Data are means ± SD; n = 5 biological replicates. Different lowercase letters indicate statistically significant differences by one-way ANOVA followed by Duncan’s multiple-range test (P < 0.05). Source data of (b, e, i, j) are provided as a Source Data file.
The C-terminal 340 amino acids in the WD40 repeats of AtCOP1 (AtCOP1C) were previously shown to be sufficient for UV-B-dependent interaction with AtUVR82. The alignment of AtCOP1 and OsCOP1 protein sequences indicated that the two proteins are highly similar over their C termini but differ in their N termini (Supplementary Fig. 5a). In Y2H assays, OsCOP1C (the C-terminal 340 amino acids of OsCOP1) indeed strongly interacted with AtUVR8 and OsUVR8a, like AtCOP1C (Fig. 6b and Supplementary Fig. 7b), suggesting that the C terminus, rather than the N terminus of COP1 is potentially a conserved domain involved in the interaction between UVR8s and COP1. To validate this idea, we generated chimeric proteins in which the C terminus of AtCOP1 was replaced with that from OsCOP1 (Swap_At1) or the C terminus of OsCOP1 was replaced with that from AtCOP1 (Swap_Os) (Fig. 6c), to analyze their capacity to interact with AtUVR8. In LCI assays, UV-B light induced the interaction of Swap_At1 with AtUVR8. However, we detected no interaction between Swap_Os and AtUVR8 regardless of UV-B light exposure (Fig. 6d). In Y2H assays, Swap_At1 interacted with AtUVR8R338A, a monomeric UVR8 variant that constitutively interacts with COP134, although to a lesser extent than AtCOP1. However, as with OsCOP1, Swap_Os did not interact with AtUVR8R338A (Fig. 6e). These results suggest that in the regulation of the UVR8–COP1 interaction (Supplementary Fig. 7c), the C terminus of OsCOP1 plays a positive role that is conserved with respect to AtCOP1, whereas the N terminus of OsCOP1 may play a negative and unique role.
A comprehensive phylogenetic analysis of COP1 indicated that COP1 orthologs are widely present, with a clear separation between dicots and monocots (Fig. 6f). A multiple protein sequence alignment among COP1 orthologs showed that their C termini are more conserved than their N termini (Supplementary Fig. 8). Therefore, we tested whether the N-terminal domains of these monocot COP1s affected the UVR8 interaction as that of OsCOP1 did. In LCI and Y2H assays, the interaction of sorghum (Sorghum bicolor, Sb) COP1 with SbUVR8 and the interaction of barley (Hordeum vulgare, Hv) COP1 with HvUVR8s were much weaker than that of AtCOP1 with AtUVR8 (Supplementary Fig. 9). The interaction of HvCOP1 or SbCOP1 with AtUVR8 was also barely detectable (Fig. 6g). When the N-terminal domain of HvCOP1 or SbCOP1 was replaced with the N terminus of AtCOP1, Swap_At2 (HvCOP1 with the N terminus of AtCOP1) and Swap_At3 (SbCOP1 with the N terminus of AtCOP1) interacted well with AtUVR8R338A, like AtCOP1 did. However, replacing the N terminus of AtCOP1 with that of HvCOP1 (Swap_Hv) or SbCOP1 (Swap_Sb) disrupted the interaction of these chimeric proteins with UVR8 (Fig. 6h–j). Collectively, these results suggest the differentiation in UVR8–COP1 interaction modes between Arabidopsis and monocots.
Transcriptome responses to UV-B light vary between Arabidopsis and rice
The finding that Arabidopsis and rice possess distinct UVR8–COP1 interaction modes prompted us to investigate whether their UV-B signaling pathways differ at a global level. We used transcriptome deep sequencing (RNA-seq) to analyze the transcriptome responses to UV-B light in Arabidopsis and rice, using WT seedlings (Col-0 for Arabidopsis and DJ for rice) grown under WL conditions alone or supplemented with UV-B light. We identified 262 and 541 differentially expressed genes (DEGs) as being regulated by UV-B in Arabidopsis and rice, respectively, using a minimum absolute log2(FC) value of 1 in transcript levels with a P < 0.05 as criteria (Supplementary Datas 1 and 2). There were 113 genes upregulated and 149 genes downregulated in response to UV-B treatment in Arabidopsis, and 265 genes upregulated and 276 genes downregulated in rice (Supplementary Fig. 10a).
Gene ontology (GO) term enrichment analysis for biological processes revealed that the genes upregulated by UV-B exposure in Arabidopsis are enriched in various biosynthetic and metabolic processes and responses to abiotic stimuli, while the genes downregulated by UV-B treatment were predominantly enriched in various stress responses (Fig. 7a). Quite differently, in rice, both sets of genes were enriched in biosynthetic and metabolic processes as well as stress responses. Most UV-B-upregulated genes were involved in stress responses, while most UV-B-downregulated genes were related to stress responses, protein phosphorylation, and metabolic processes (Fig. 7a). We also performed a kyoto encyclopedia of genes and genomes pathway enrichment analysis and determined that UV-B-regulated genes in Arabidopsis were enriched in a series of biosynthetic and metabolic processes (Supplementary Fig. 10b). In rice, UV-B-regulated genes were involved not only in biosynthetic and metabolic processes, but also in mitogen-activated protein kinase signaling and plant–pathogen interactions, both of which regulate plant defense responses (Supplementary Fig. 10b). These results suggest that UV-B light primarily promotes growth-related gene expression but represses stress-related gene expression in Arabidopsis, with an opposite regulatory pattern existing in rice. To test this hypothesis, we examined the expression levels of several genes involved in leucine biosynthesis for plant defense responses, namely Arabidopsis ISOPROPYLMALATE DEHYDROGENASE 1 (AtIMD1), 2-ISOPROPYLMALATE SYNTHASE 2 (AtIMS2), and METHYLTHIOALKYLMALATE SYNTHASE 1 (AtMAM1), as well as their putative rice orthologs Os03g0655700, Os12g0138900, and Os11g0142500. We observed that AtIMD, AtIMS2, and AtMAM1 expression was induced by UV-B light in a UVR8-dependent manner, whereas that of Os03g0655700 and Os12g0138900 was not (Fig. 7b and Supplementary Fig. 10c). Os11g0142500 was induced by UV-B light at a much lower level than AtMAM1 (Supplementary Fig. 10c).
a Top 10 enriched GO terms in the category of biological processes for genes upregulated (top) or downregulated (bottom) by UV-B light in Arabidopsis (left) and rice (right). b qRT-PCR analysis of the relative transcript levels of AtIMD1 and AtIMS2 in Col-0 and uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Transcript levels were normalized to ACTIN, with the transcript levels in Col-0 under −UV-B set to 1. qRT-PCR analysis of the transcript levels of Os03g0655700 and Os12g0138900 in WT DJ and Osuvr8ab seedlings grown under WL or WL + UV-B. Transcript levels were normalized to UBQ, with the transcript levels in WT DJ under WL set to 1. Data are means ± SD; n = 3 biological replicates. c Percentage of UV-B-regulated rice genes with (pink) or without (purple) homologs in Arabidopsis. d qRT-PCR analysis of the relative transcript levels of At1g03010 and AtSETH6 in Col-0 and uvr8-6 seedlings grown under −UV-B or +UV-B conditions. Transcript levels were normalized to ACTIN, with transcript levels in Col-0 under −UV-B set to 1. qRT-PCR analysis of the relative transcript levels of Os03g0347700 in WT DJ and Osuvr8ab seedlings grown under WL or WL + UV-B conditions. Transcript levels were normalized to UBQ, with the transcript levels in WT DJ under WL set to 1. Data are means ± SD; n = 3 biological replicates. e Top five enriched GO terms in the category of biological processes for UV-B-regulated specific genes in rice. f qRT-PCR analysis of the relative transcript levels of OsRab16D, OsRAG2, OsPBZ1, and OsOCPI2 in WT DJ and Osuvr8ab seedlings grown under WL or WL + UV-B. Transcript levels were normalized to UBQ, with the transcript levels in WT DJ under WL set to 1. Data are means ± SD; n = 3 biological replicates. Source data of (b, d, f) are provided as a Source Data file.
Notably, we determined that only 29.9% of UV-B-regulated genes in rice have orthologs in Arabidopsis, indicating that 70.1% are rice-specific UV-B-regulated genes (Fig. 7c and Supplementary Data 3). To verify this notion, we examined the expression of Os03g0347700, encoding a BTB/POZ domain-containing protein, by qRT-PCR analysis: we confirmed its UV-B light-dependent induction via OsUVR8a and OsUVR8b, while finding that its Arabidopsis orthologs At1g03010 and AtSETH6 showed little induction by UV-B (Fig. 7d). The transcription factor gene WRKY DNA-BINDING PROTEIN 70 (OsWRKY70) was downregulated by UV-B light via OsUVR8s, as was its Arabidopsis ortholog AtWRKY33 via AtUVR8, although to a lesser degree and in a UVR8-independent manner (Supplementary Fig. 10d). A GO term enrichment analysis of rice-specific UV-B-regulated genes showed that they are largely enriched in stress-related processes (Fig. 7e), including the dehydrin gene OsRab16D, the α-amylase/trypsin inhibitor gene RICE ALBUMIN GENE 2 (OsRAG2), PROBENAZOLE-INDUCED PROTEIN 1 (OsPBZ1) and CHYMOTRYPSIN PROTEASE INHIBITOR GENE (OsOCPI2). OsRab16D and OsRAG2 expression was induced by UV-B light, while that of OsPBZ1 and OsOCPI2 was repressed by UV-B light. These effects were diminished or even reversed in the Osuvr8ab mutants (Fig. 7f). These results suggest that UV-B light provides an informational signal that globally governs stress-related gene expression via OsUVR8s in rice. Furthermore, we performed an enrichment analysis for transcription factor binding sites (TFBS) in the promoters of UV-B-responsive genes, based on our RNA-seq data, to identify candidate transcription factor families involved in the regulation of UV-B-responsive gene expression downstream of OsUVR8s. Multiple conserved transcription factor families shared a role in rice and Arabidopsis in regulating UV-B responsive gene expression, including MYB, basic helix-loop-helix (bHLH), and basic leucine zipper (bZIP) family members (Supplementary Fig. 10e, f). Therefore, we propose that UVR8 may mediate UV-B-responsive gene expression in concert with transcription factors, which is possibly conserved in rice and Arabidopsis (Fig. 8).
In response to UV-B light (left panel), monomerization of the Arabidopsis UV-B receptor AtUVR8 leads to the nuclear accumulation of active AtUVR8, which interacts with AtCOP1 and other signaling factors including MYB and WRKY transcription factors, resulting in the expression of growth- and stress-related genes and regulation of plant development and acclimation. The rice UV-B receptors OsUVR8a and OsUVR8b are constitutively present in the nucleus via their NLS; in response to UV-B light (right panel), OsUVR8a and OsUVR8b also monomerize and activate transcription of growth- and stress-related genes to regulate plant development and acclimation. However, OsUVR8a and OsUVR8b accumulate in the nucleus independently of UV-B light and OsCOP1, and may mediate UV-B responsive gene expression in concert with transcription factors such as MYB and basic helix-loop-helix (bHLH) family members. OsUVR8a and OsUVR8b have weak interaction with OsCOP1 which is a repressor in rice UV-B responses. UV-B light predominantly upregulates the expression of growth-related genes and downregulates that of stress-related genes in Arabidopsis, whereas these transcriptional patterns are reversed in rice.
Discussion
UV-B light can be perceived and utilized by plants as an informational signal to mediate developmental and acclimatory responses through the UVR8 photoreceptor signaling pathway1. The presence of UVR8 orthologs in various members of the green lineage, from green algae to angiosperms, suggests the conservation of UV-B signaling pathways initiated by UVR82,23. Indeed, the reported UVR8 orthologs including CrUVR8, MpUVR8, and SlUVR8 share functional similarity with AtUVR8 in the regulation of light-induced development and/or stress tolerance24,26,27,28,35. They mediate UV-B signaling in a conserved manner, principally relying on at least one of the following three key molecular events upon UV-B exposure: (1) UVR8 monomerization; (2) the UVR8–COP1 interaction; and (3) the nuclear accumulation of UVR81. UV-B light was reported to inhibit the growth of japonica rice coleoptiles and induce anthocyanin accumulation in purple rice29. However, how UV-B light regulates rice growth and stress tolerance at later developmental stages is not clear. In addition, there is no information as to whether specific rice UV-B photoreceptors are involved in these regulatory steps.
In this study, we demonstrate that UV-B light plays an important role in rice seedling architecture and stress acclimation, by controlling seedling height, increasing stem thickness, and promoting stress tolerance against UV-B stress (Figs. 1 and 8 and Supplementary Fig. 1). Therefore, rice seedling morphogenesis can be utilized as a monocot UV-B response model, to monitor the early effects of UV-B light on rice cultivation. Considering the trade-off between growth and defense, it is of significant interest to study whether OsUVR8a and OsUVR8b contribute to the regulation of yield traits, in addition to their role in shaping seedling architecture and stress acclimation. Unlike chlorophyte green algae and some eudicots containing single-copy UVR8 orthologs22,24, the rice genome harbors two UVR8 orthologs, OsUVR8a and OsUVR8b, likely the result of whole-gene duplication events. This increase in UVR8 copy number not only reflects rice's long-term adaptation to relatively high UV-B radiation in terrestrial environments, but also suggests potential functional redundancy and specialization between OsUVR8a and OsUVR8b. They both contain the conserved tryptophans for UV-B perception, and arginines for dimer stabilization in the absence of UV-B light (Fig. 1a and Supplementary Fig. 1f). As experimentally verified in this study, OsUVR8a and OsUVR8b both undergo UV-B-activated monomerization, as does AtUVR8 (Fig. 2i), and localize in the nucleus in a pattern that is not affected by UV-B light or a mutation in the Zn-binding RING-finger domain of OsCOP1 (Figs. 2j and 5h). However, it is apparent that OsUVR8a and OsUVR8b display functional specialization in mediating UV-B responses. Compared to OsUVR8a, OsUVR8b is more effective in producing shorter seedlings with thicker stems, UV-B stress tolerance and high expression of UV-B-responsive genes when overexpressed in rice (Fig. 2 and Supplementary Fig. 2). Notably, the fraction of OsUVR8b present as a monomer is greater than that for AtUVR8 and OsUVR8a in the absence of UV-B light (Fig. 2i and Supplementary Fig. 11). This might allow OsUVR8b to interact with other effectors rather than OsCOP1 to mediate the observed constitutive physiological responses independent of UV-B light. On the other hand, OsUVR8a plays a predominant role when introduced in the Arabidopsis uvr8-6 mutant in terms of UV-B-induced photomorphogenesis and stress acclimation (Fig. 3). OsUVR8a shows higher affinity for AtCOP1 than OsUVR8b, although the affinity of AtUVR8 for AtCOP1 is much greater (Fig. 3f–h). The molecular and biochemical basis by which OsUVR8a and OsUVR8b function differentially awaits further investigations.
UV-B-induced nuclear accumulation is a key determinant of AtUVR8 activity to mediate UV-B light signaling. This nuclear accumulation requires the direct interaction of AtCOP1 with AtUVR8 to promote AtUVR8 nuclear retention6,7,15. In this study, we demonstrate that AtUVR8, OsUVR8a and OsUVR8b all localize to the nucleus through a typical NLS in their C termini, although some AtUVR8 is also present in the cytoplasm in the absence of UV-B light (Fig. 4a). In the presence of UV-B light, AtCOP1 activity compensates for an inactivating mutation of the AtUVR8 NLS, which would otherwise exclude AtUVR8 from the nucleus, to accomplish normal UV-B-induced photomorphogenesis (Fig. 4). However, the nuclear localization of OsUVR8a and OsUVR8b is independent of UV-B light (Fig. 3i, j and Supplementary Fig. 3) and is not impaired by the mutation in the RING finger of OsCOP1 (Fig. 5a–f and Supplementary Fig. 5h). Therefore, the mechanism regulating UVR8 nuclear dynamics is not conserved between Arabidopsis and rice. Consistent with the lack of requirement for OsCOP1 in the nuclear localization of OsUVR8a and OsUVR8b, OsCOP1 may not play an essential role, in contrast to AtCOP1, in UV-B-regulated seedling growth. Admittedly, the functional contributions of OsCOP1 to the above physiological and cellular responses are based on a weak mutant allele of OsCOP1 encoding a protein that lacks a cysteine residue (Cys-96) in the cysteine cluster crucial for its E3 ligase activity within the Zn-binding RING-finger domain. The embryo lethality observed with a strong Oscop1 allele limited a thorough characterization of the functionality of OsCOP1 in the rice UV-B responses. The exact role of OsCOP1 needs further investigation based on viable and yet strong mutant alleles. At the molecular level, OsCOP1 shows rather low affinity with AtUVR8 (Supplementary Fig. 6c), probably because its N terminus disrupts its interaction with UVR8 (Fig. 6 and Supplementary Fig. 8). Such an interaction mode appears to be conserved in monocot plants, as supported by the low sequence identity between the N termini of AtCOP1 and that of monocot COP1 orthologs (Supplementary Fig. 8). In terms of transcriptome responses, UV-B light predominantly promotes development and growth in Arabidopsis, but mainly affects stress-related gene expression in rice (Fig. 7). During plant terrestrialization, the ability to perceive UV-B light and decipher this signal to mount a stress response was likely positively selected in plants, driven by the increase in UV-B irradiation they were exposed to on land. Arabidopsis and rice might have experienced adaptive variation in the canonical UVR8–COP1 signaling pathway, including distinct requirements and roles for COP1. It is also possible that their UV-B signaling mechanisms diversified through increasing crosstalk with other pathways. The identification of conserved or rice-specific UV-B signaling regulators and their underlying mechanism will be achieved by further analyses such as the establishment of OsUVR8a and OsUVR8b interactomes.
Methods
Plant materials and growth conditions
The WT Arabidopsis (A. thaliana) accession used in this study was Columbia (Col-0). The following mutants and transgenic lines used in this study were described previously: cop1-436, uvr8-611, YFP-AtUVR8/uvr8-634, YFP-AtUVR8/cop1-417, and YFP-AtCOP1/cop1-412,15. The transgenic lines YFP-mAtUVR8/uvr8-6, CFP-OsUVR8a/uvr8-6, CFP-OsUVR8b/uvr8-6, CFP-mOsUVR8a/uvr8-6, CFP-mOsUVR8b/uvr8-6, and CFP-OsCOP1/cop1-4 were generated in Arabidopsis by the floral dip method37 using Agrobacterium (Agrobacterium tumefaciens) strain GV3101. CFP-OsUVR8a/cop1-4 and CFP-OsUVR8b/cop1-4 were generated by crossing.
Arabidopsis seeds were surface sterilized and sown on Murashige and Skoog (MS) medium solidified with 0.8% (w/v) agar and supplemented with 1% (w/v) sucrose for molecular and biochemical assays, or with 0.3% (w/v) sucrose for phenotypic analysis, and then stratified at 4 °C for 4 days in the dark before light treatment. For UV-B-mediated photomorphogenesis, seedlings were grown at 22 °C under continuous low-intensity WL (3 μmol m−2 s−1; measured by an HR-350 Light Meter, Hipoint) supplemented with UV-B light (7 μW cm−2, equivalent to ~1.05 μmol m−2 s−1; measured by a UV-297 UV-B Light Meter, HANDY) provided by Philips TL20W/01RS narrowband UV-B tubes11 under a 350-nm cutoff (half-maximal transmission at 350 nm) ZUL0350 filter (−UV-B; Asahi Spectra) or a 300-nm cutoff (half-maximal transmission at 300 nm) ZUL0300 filter (+UV-B; Asahi Spectra).
The WT rice (O. sativa) cultivars used in this study were Nip and DJ. The overexpression rice lines used in this study were in the Nip background. All constructs were individually transformed into Agrobacterium strain EHA105, and a tissue-culture-based method was used to transform Nip38 (Frontier Laboratories of Systems Crop Designs Co., Ltd, Beijing, China). A CRISPR/Cas9 genome editing system39 was used to generate the knockout mutants in DJ. The double mutant Osuvr8a Osuvr8b (Osuvr8ab) was generated using single guide RNAs (sgRNAs) per gene, targeting the second exon of OsUVR8a or OsUVR8b; the Oscop1 mutants were generated using a sgRNA targeting the first exon of OsCOP140. Mutations were confirmed by Sanger sequencing of PCR products in the T0 and T1 generations.
Dehusked rice caryopses were surface sterilized for 2 min in 70% (v/v) ethanol and for 20 min in 50% (v/v) bleach. After being washed with deionized water, the disinfected caryopses were sown onto MS medium solidified with 0.8% (w/v) agar and supplemented with 1% (w/v) sucrose before being placed in darkness at 37 °C for 3 days to induce germination. The resulting young seedlings were then transferred to a growth chamber at 28 °C for 7 days under short-day conditions (10-h light/14-h dark photoperiod) in WL alone (200 μmol m−2 s−1, produced by LEDs from SANANBIO, China) or WL supplemented with narrowband UV-B light (15 μW cm−2, equivalent to ~2.25 μmol m−2 s−1; measured by a UV-297 UV-B Light Meter, HANDY).
BLAST search and phylogenetic analysis
Reciprocal BLASTP searches were conducted on the NCBI website (http://blast.ncbi.nlm.nih.gov). The evolutionary history of COP1 proteins was inferred using the neighbor-joining method41. The optimal tree with the sum of branch length = 0.88094058 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches42. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method43 and are in the units of the number of amino acid substitutions per site. This analysis involved the amino acid sequence of 12 COP1 proteins. All positions containing gaps and missing data were eliminated (complete deletion option), retaining 641 positions in the final dataset. The evolutionary analysis was conducted in MEGA X44.
Protein sequence alignment and structural modeling
Protein sequence alignments were performed in MEGA X and Genedoc. The full-length protein sequences of OsUVR8a and OsUVR8b were submitted to the web-based three-dimensional structure prediction server SWISS-MODEL using AtUVR8 as a template (PDB ID 4D9SA). The PyMOL Molecular Graphics System (Schrödinger) was used to visualize and refine the obtained three-dimensional AtUVR8, OsUVR8a, and OsUVR8b structures.
Plasmid construction and site-directed mutagenesis
To obtain transgenic plants expressing CFP-OsUVR8a or CFP-OsUVR8b, the full-length OsUVR8a and OsUVR8b coding sequences were individually cloned into the pCAMBIA2300 binary vector using SalI and BamHI restriction sites. Similarly, the full-length OsCOP1 coding sequence was cloned into pCAMBIA2300 at the SalI and SacI restriction sites. Site-directed mutagenesis by PCR was used to generate pEarleyGate 104-YFP-mAtUVR8, pCAMBIA2300-CFP-mOsUVR8a, and pCAMBIA2300-CFP-mOsUVR8b constructs encoding proteins with a mutated NLS, using pEarleyGate 104-YFP-AtUVR834, pCAMBIA2300-CFP-OsUVR8a, and pCAMBIA2300-CFP-OsUVR8b as templates, respectively.
For the Y2H assays, the full-length OsUVR8a, OsUVR8b, SbUVR8, HvUVR8a, and HvUVR8b coding sequences were individually cloned into the pLexA vector (Clontech) using EcoRI and XhoI restriction sites. The coding sequences of full-length OsCOP1, HvCOP1, and SbCOP1, truncated OsCOP1C, and chimeric Swap_At1, Swap_At2, Swap_At3, Swap_Os, Swap_Hv, and Swap_Sb were individually cloned into the pB42AD vector (Clontech) at the EcoRI and XhoI restriction sites. The pLexA-AtUVR8, pB42AD-AtCOP1, and pB42AD-AtCOP1C constructs were used as described previously34,45.
For the firefly LCI assays, the full-length AtUVR8, OsUVR8a, OsUVR8b, SbUVR8, HvUVR8a, and HvUVR8b coding sequences were individually cloned into the modified pCAMBIA1300-nLuc46 vector with a start codon using KpnI and SalI restriction sites. The full-length OsCOP1 and SbCOP1 coding sequence was cloned into pCAMBIA1300-cLuc46 at the KpnI and SalI restriction sites. The full-length coding sequences of HvCOP1 and chimeric Swap_At1 and Swap_Os were also individually cloned into pCAMBIA1300-cLuc46 at the BamHI and SalI restriction sites. pCAMBIA1300-cLuc-AtCOP1 was used as described previously18.
All primers are listed in Supplementary Data 4, and all constructs were confirmed by Sanger sequencing.
Measurements of hypocotyl length, whole-plant height, and lengths of first and second-leaf sheaths in rice
For each Arabidopsis line grown under −UV-B or +UV-B conditions for 4 days, the hypocotyl length of at least 20 Arabidopsis seedlings was measured. Length was quantified with ImageJ software (https://imagej.nih.gov/ij/). For each rice line grown under short-day conditions in WL alone or WL supplemented with UV-B light for 7 days, the height of the whole seedling and the lengths of the first and second leaf sheaths of at least ten rice seedlings were measured with ImageJ software.
Tissue slicing and microscopy
To measure the cross-sectional area of rice stems, stem segments from 10-day-old rice seedlings were collected and embedded in 7% (w/v) agarose. After solidification, the samples were sliced into 80-μm transverse sections using a Leica VT1000S microtome. The median sections were stained with 1 g/L toluidine blue and visualized using a Nikon-NiU dissecting microscope. Cross-sectional areas were measured with ImageJ software.
UV-B stress assays
Four-day-old Arabidopsis seedlings grown under WL (50 μmol m−2 s−1) were kept under WL alone (non-acclimated) or transferred to WL supplemented with narrowband UV-B light (7 μW cm−2; equivalent to ~1.05 μmol m−2 s−1) (acclimated) for 4 days. All seedlings were then treated with WL (50 μmol m−2 s−1) supplemented with UV-B stress (200 μW cm−2; equivalent to ~30.00 μmol m−2 s−1) produced by Philips TL40W/12RS broadband UV-B tubes11 for 2 days and allowed to recover under WL (50 μmol m−2 s−1) for 3 days, at which time the survival rate of each line was determined.
Ten-day-old rice seedlings were grown under short-day conditions in WL (200 μmol m−2 s−1) alone (non-acclimated) or WL supplemented with narrowband UV-B light (15 μW cm−2; equivalent to ~2.25 μmol m−2 s−1) (acclimated). All seedlings were then treated with WL (200 μmol m−2 s−1) supplemented with UV-B stress (250 μW cm−2; equivalent to ~37.50 m−2 s−1) for 2 days and allowed to recover under WL (200 μmol m−2 s−1) for 1 day.
RNA extraction and reverse-transcription quantitative PCR
Total RNA was extracted from 4-day-old Arabidopsis seedlings or 10-day-old rice seedlings using an Eastep Super Total RNA Extraction Kit (#LS1040, Promega). Total RNA was reverse transcribed into first-strand cDNA using a GoScript Reverse Transcription System (#A2791, Promega). Quantitative PCR (qPCR) analysis was performed using iTaq Universal SYBR Green Supermix (#1725125, Bio-Rad) on a CFX Connect Real-Time PCR System (Bio-Rad). Each experiment was performed with three independent samples, and qPCR reactions were performed in three technical replicates for each sample. The primers used for qPCR are listed in Supplementary Data 4.
Confocal microscopy
Confocal laser scanning microscopy was performed using a Zeiss LSM780 and LSM900 microscope. YFP, CFP 4′,6-diamidino-2-phenylindole (DAPI), and mCherry fluorescence was excited at 514 nm, 458 nm, 405 nm, and 561 nm, respectively. The emission of YFP fluorescence was collected at 519–550 nm, that of CFP at 463–513 nm, that of DAPI at 410–460 nm, and that of mCherry at 606–616 nm. Images were acquired using ZEN software (Zeiss). To stain nuclei, seedlings were mounted in water containing 10 μg/mL DAPI (#D9542, Sigma-Aldrich) under −UV-B light or +UV-B light conditions and incubated for 12 h before imaging. For transfection, rice protoplasts were prepared as previously described47 with slight modifications. The stems and sheaths from 14-day-old WT Nip or Oscop1 mutant seedlings grown under continuous darkness were used to prepare protoplasts. The pCAMBIA2300-CFP-OsUVR8a or pCAMBIA2300-CFP-OsUVR8b and pCAMBIA1300-mCherry-H2B plasmids were co-transfected into rice protoplasts by polyethylene glycol-mediated transfection. Fluorescence was observed 10–12 h later.
Y2H assays
The appropriate combinations of fusion constructs carrying the B42 activation domain (AD) or the LexA DNA-binding domain (BD) were co-transformed into yeast (Saccharomyces cerevisiae) strain EGY48 containing the reporter plasmid p8op-LacZ according to the instructions provided with the Matchmaker LexA Two-Hybrid System (#K1609-1, Clontech). Transformants were selected on minimal synthetic defined (SD) medium lacking His, Trp, and Ura (SD/−His/−Trp/−Ura). β-Galactosidase activity was analyzed using o-nitrophenyl-β-D-galactopyranoside as a substrate (β-galactosidase units = 1000 × OD420/[t × V × OD600], where t is elapsed time [in min] of incubation, V = 0.1 mL × concentration factor, and OD600 = A600 of 1 mL of culture). At least four independent experiments were performed for each interaction pair. Detection of BD-fusion and AD-fusion proteins in Y2H assays is shown in Supplementary Fig. 12.
Firefly LCI assay
Firefly LCI was performed in the leaves of N. benthamiana plants as described previously46. Briefly, Agrobacterium cells (strain GV2260) carrying the appropriate nLUC or cLUC fusion construct were individually resuspended in infiltration buffer (10 mM MgCl2, 10 mM MES (pH 5.7), 200 µM acetosyringone) at an OD600 of 1 and mixed at a 1:1 (v/v) ratio before infiltration into N. benthamiana leaves. After infiltration, N. benthamiana plants were grown for 2 days before the infiltrated leaves were further infiltrated with 100 μM luciferin, and imaged using a Tanon 5200 S Luminescent Imaging Workstation. Three independent experiments were performed for each construct pair, with three infiltrated samples assayed per experiment. The detection of cLuc-fusion and nLuc-fusion proteins in LCI assays is shown in Supplementary Fig. 13.
Nuclear fractionation assays
Nuclear fractionation assays were performed as previously described6,7 with slight modifications. 4-day-old Arabidopsis and 10-day-old rice seedlings were homogenized in Honda buffer containing 2.5% (w/v) Ficoll 400, 5% (w/v) dextran T40, 0.4 M sucrose, 25 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride (PMSF) and complete protease inhibitor cocktail (#04693132001, Roche) and filtered through one layer of Miracloth (#3417361, Merck Millipore). After Triton X-100 addition to a final concentration of 0.5% (v/v), the homogenate was incubated on ice for 15 min and centrifuged at 1500 × g for 5 min at 4 °C. The supernatant was collected as the cytosolic fraction. The pellet was washed twice with Honda buffer containing 0.1% (v/v) Triton X-100, and then resuspended gently in 1 mL Honda buffer and centrifuged at 1500 × g for 5 min at 4 °C to pellet the nuclei.
Co-IP and immunoblotting
Total protein was extracted from 4-day-old Arabidopsis or 10-day-old rice seedlings in protein extraction buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% (v/v) glycerol, 0.1% (v/v) Tween 20, 1 mM PMSF and complete protease inhibitor cocktail. Five milligrams of total proteins were incubated with 10 μL of anti-GFP antibody (#G10362, Thermo Fisher Scientific) coupled with 50 μL of Dynabeads Protein G (#10004D, Thermo Fisher Scientific) for 3 h at 4 °C under the same conditions in which the seedlings were grown. The Dynabeads were washed three times with protein extraction buffer, and the precipitates were eluted with 100 mM glycine (pH 2.5) and 100 mM NaCl, immediately neutralized by addition of 2 M Tris-HCl (pH 9.0) and 100 mM NaCl. and then concentrated using Strataresin (Stratagene) before immunoblot analysis. Three independent experiments were performed and one representative result is presented.
Immunoblotting was performed as previously described18. The following primary antibodies used in this study are commercially available or were described previously: anti-GFP (1:3000 dilution; #1181446001, Roche), anti-UVR812 (1:200 dilution), anti-PEPC (1:3000 dilution; #ab34793, Abcam), anti-RPN618 (1:3000 dilution), anti-LexA (1:3000 dilution; #ab14533, Abcam), anti-HA (1:10,000 dilution; #26183, Thermo Fisher Scientific) and anti-Luc (1:3000 dilution; #L0159, Sigma-Aldrich). Anti-OsUVR8s antibodies were generated for this study by raising rabbit polyclonal antibodies against full-length recombinant His-UVR8 proteins.
RNA-seq analysis
DJ rice seedlings were grown under short-day conditions in WL (200 μmol m−2 s−1) alone or WL supplemented with narrowband UV-B light (15 μW cm−2; equivalent to ~2.25 μmol m−2 s−1) for 7 days. Col-0 Arabidopsis seedlings were grown under −UV-B (3 μmol m−2 s−1 WL) or +UV-B (3 μmol m−2 s−1 WL supplemented with 7 μW cm−2 UV-B light; equivalent to ~1.05 μmol m−2 s−1) conditions for 4 days. Seedling shoots were harvested for RNA extraction. Total RNA was extracted using an RNeasy Plant Mini Kit (#74904, Qiagen). Deep sequencing of mRNA was performed using a HiSeq X Ten instrument (Annaroad Gene Technology (Beijing) Co., Ltd.). Three biological replicates per genotype and condition were prepared for sequencing. The reads were mapped to the IRGSP-1.0 and TAIR 10 reference genome, respectively, using the spliced alignment identification tool TopHat. The sequence alignment files generated by TopHat were then used as input for Cufflinks, which assembles the mapped files into transcripts. Significant DEGs were identified by Cuffdiff, which estimated FPKM (fragments per kilobase of transcript per million fragments mapped) values48.
TFBS enrichment analysis
The upstream 2-kb promoter regions of UV-B-regulated genes were extracted using in-house scripts. TFBS scanning of Arabidopsis and rice promoters were conducted by PlantPan v4.049.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the article and its Supplementary Information files. Rice and Arabidopsis sequence data from this study can be found in the NCBI database under BioProject codes PRJNA1012538 and PRJNA1140725 and Sequence Read Archive (SRA) IDs SRR25997685-SRR25997690 and SRR30010007-SRR30010012. Source data are provided with this paper.
References
Podolec, R., Demarsy, E. & Ulm, R. Perception and signaling of ultraviolet-B radiation in plants. Annu Rev. Plant Biol. 72, 793–822 (2021).
Rizzini, L. et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106 (2011).
Wu, D. et al. Structural basis of ultraviolet-B perception by UVR8. Nature 484, 214–219 (2012).
Christie, J. M. et al. Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335, 1492–1496 (2012).
Kaiserli, E. & Jenkins, G. I. UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19, 2662–2673 (2007).
Qian, C. et al. Dual-Source Nuclear monomers of UV-B light receptor direct photomorphogenesis in Arabidopsis. Mol. Plant 9, 1671–1674 (2016).
Yin, R., Skvortsova, M. Y., Loubery, S. & Ulm, R. COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc. Natl. Acad. Sci. USA 113, E4415–E4422 (2016).
Hoecker, U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr. Opin. Plant Biol. 37, 63–69 (2017).
Lau, K., Podolec, R., Chappuis, R., Ulm, R. & Hothorn, M. Plant photoreceptors and their signaling components compete for COP1 binding via VP peptide motifs. EMBO J. 38, e102140 (2019).
Ponnu, J., Riedel, T., Penner, E., Schrader, A. & Hoecker, U. Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc. Natl. Acad. Sci. USA 116, 27133–27141 (2019).
Favory, J. J. et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28, 591–601 (2009).
Huang, X. et al. Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc. Natl. Acad. Sci. USA 110, 16669–16674 (2013).
Yin, R., Arongaus, A. B., Binkert, M. & Ulm, R. Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell 27, 202–213 (2015).
Wang, Y. et al. Structural insight into UV-B-activated UVR8 bound to COP1. Sci. Adv. 8, eabn3337 (2022).
Fang, F. et al. Mechanisms of UV-B light-induced photoreceptor UVR8 nuclear localization dynamics. N. Phytol. 236, 1824–1837 (2022).
Gruber, H. et al. Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107, 20132–20137 (2010).
Ouyang, X. et al. Coordinated photomorphogenic UV-B signaling network captured by mathematical modeling. Proc. Natl. Acad. Sci. USA 111, 11539–11544 (2014).
Ren, H. et al. Two E3 ligases antagonistically regulate the UV-B response in Arabidopsis. Proc. Natl. Acad. Sci. USA 116, 4722–4731 (2019).
Heijde, M. & Ulm, R. Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc. Natl. Acad. Sci. USA 110, 1113–1118 (2013).
Heilmann, M. & Jenkins, G. I. Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV RESISTANCE LOCUS8 in intact Arabidopsis plants. Plant Physiol. 161, 547–555 (2013).
Wang, L. et al. RUP2 facilitates UVR8 redimerization via two interfaces. Plant Commun. 4, 100428 (2023).
Han, X. et al. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol. Plant 12, 847–862 (2019).
Zhang, Z. et al. Origin and adaptive evolution of UV RESISTANCE LOCUS 8-mediated signaling during plant terrestrialization. Plant Physiol. 188, 332–346 (2022).
Tilbrook, K. et al. UV-B perception and acclimation in Chlamydomonas reinhardtii. Plant Cell 28, 966–983 (2016).
Soriano, G. et al. Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. N. Phytol. 217, 151–162 (2018).
Liu, X. et al. Pivotal roles of tomato photoreceptor SlUVR8 in seedling development and UV-B stress tolerance. Biochem. Biophys. Res. Commun. 522, 177–183 (2020).
Kondou, Y. et al. Physiological function of photoreceptor UVR8 in UV-B tolerance in the liverwort Marchantia polymorpha. Planta 249, 1349–1364 (2019).
Allorent, G. et al. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 113, 14864–14869 (2016).
Idris, M. et al. UV-B signalling in rice: response identification, gene expression profiling and mutant isolation. Plant Cell Environ. 44, 1468–1485 (2021).
Tsuge, T. et al. Phytochrome-mediated control of COP1 gene expression in rice plants. Mol. Genet. Genomics 265, 43–50 (2001).
Tanaka, N. et al. The COP1 ortholog PPS regulates the juvenile-adult and vegetative-reproductive phase changes in rice. Plant Cell 23, 2143–2154 (2011).
Seo, H. S., Watanabe, E., Tokutomi, S., Nagatani, A. & Chua, N. H. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18, 617–622 (2004).
Oravecz, A. et al. CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18, 1975–1990 (2006).
Huang, X., Yang, P., Ouyang, X., Chen, L. & Deng, X. W. Photoactivated UVR8-COP1 module determines photomorphogenic UV-B signaling output in Arabidopsis. PLoS Genet. 10, e1004218 (2014).
Li, H. et al. Tomato UV-B receptor SlUVR8 mediates plant acclimation to UV-B radiation and enhances fruit chloroplast development via regulating SlGLK2. Sci. Rep. 8, 6097 (2018).
McNellis, T. W. et al. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6, 487–500 (1994).
Weigel, D. et al. Activation tagging in Arabidopsis. Plant Physiol. 122, 1003–1013 (2000).
Meng, X. et al. Construction of a genome-wide mutant library in rice using CRISPR/Cas9. Mol. Plant 10, 1238–1241 (2017).
Ma, X. et al. A Robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985).
Zuckerkandl E., Pauling L. B. Evolutionary divergence and convergence in proteins. Evolv. Genes Proteins. https://doi.org/10.1016/B978-1-4832-2734-4.50017-6 (1965).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Qiu, L., Wang, X., Zhuang, G., Ouyang, X. & Huang, X. Chromosomal looping-based expression activation system in yeast. Small Methods 5, e2001135 (2021).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Zhang, Y. et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7, 30 (2011).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Chow, C. N. et al. PlantPAN 4.0: updated database for identifying conserved non-coding sequences and exploring dynamic transcriptional regulation in plant promoters. Nucleic Acids Res. 52, D1569–D1578 (2024).
Acknowledgements
We thank Yaoguang Liu (South China Agricultural University, China) for providing the pYLCRISPR/Cas9-MTmono vectors. We thank Hongtao Liu (Shenzhen University, China), Ruohe Yin (Shanghai Jiao Tong University, China) and Fang Lin (Lanzhou University, China) for sharing plant materials; Shigui Li, Peng Qin (Sichuan Agricultural University, China), Hang He (Peking University, China), and Xu Chen (Fujian Agriculture and Forestry University, China) for their suggestions; Ying Chen, Yuchao Cui, Meiling Wang, Qingfeng Liu, Jingdong Zhuang, Lei Huang, Yongying Peng, Feng Yu, Yan Liu, Xuefeng Cheng (Xiamen University, China), Jiaomei Chen, and Yongjia Zhong (Fujian Agriculture and Forestry University, China) for their technical assistance; and Wenqian Lai for her assistance with RNA-seq analysis. We thank all Huang lab members for their close teamwork. This research was supported by the National Natural Science Foundation of China (32122011 and 32070266 to X.H., and 32270250 to X.O.), the National Natural Science Foundation of Fujian (2022J02004 to X.O. and 2021J02011 to X.H.), and the Fundamental Research Funds for the Central Universities (20720220142 to X.H.). Figure 8 was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
X.H., X.O. and S.H. conceived the project. S.H. performed most of the experiments. YH.C. performed protein–protein interaction and qRT-PCR assays. YH.C., C.Q., H.R., X.L., YL.C., J.W., Y.D. and J.H. assisted in molecular experiments and plant material preparation. S.H., X.H., X.O., YH.C., C.Q. and W.T. analyzed the data. X.H. and S.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sourav Datta and the other anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, S., Chen, Y., Qian, C. et al. Nuclear accumulation of rice UV-B photoreceptors is UV-B- and OsCOP1-independent for UV-B responses. Nat Commun 15, 6396 (2024). https://doi.org/10.1038/s41467-024-50755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50755-6
This article is cited by
-
Identification of superior haplotype and haplotype combinations for UV-B stress-related genes in Rice (Oryza sativa L.)
Plant Biotechnology Reports (2025)