Abstract
Cu catalyses electrochemical CO2 reduction to valuable multicarbon products but understanding the structure-function relationship has remained elusive due to the active Cu sites being heterogenized and under dynamic re-construction during electrolysis. We herein coordinate Cu with six phenyl-1H-1,2,3-triazole derivatives to form stable coordination polymer catalysts with homogenized, single-site Cu active sites. Electronic structure modelling, X-ray absorption spectroscopy, and ultraviolet–visible spectroscopy show a widely tuneable Cu electronics by modulating the highest occupied molecular orbital energy of ligands. Using CO diffuse reflectance Fourier transform infrared spectroscopy, in-situ Raman spectroscopy, and density functional theory calculations, we find that the binding strength of *CO intermediate is positively correlated to highest occupied molecular orbital energies of the ligands. As a result, we enable a tuning of C–C coupling efficiency—a parameter we define to evaluate the efficiency of C2 production—in a broad range of 0.26 to 0.86. This work establishes a molecular platform that allows for studying structure-function relationships in CO2 electrolysis and devises new catalyst design strategies appliable to other electrocatalysis.
Similar content being viewed by others
Introduction
Electrification of chemical synthesis provides a sustainable and carbon-net-zero approach to tackling global energy and climate concerns1,2. An illustrative instance is the electrolytic process of CO2, yielding valuable multicarbon products (C2+) such as ethylene and ethanol3,4,5. Copper-based catalysts have been extensively used in catalysing the cathodic CO2 electroreduction reaction (CO2RR)6,7. It consists of multiple and branched elementary steps involving diverse intermediates adsorbed on varied sites within heterogeneous catalysts and electron/proton transfers8, making it challenging to achieve exact control over product distribution9,10.
Molecular complexes offer homogeneous catalytic sites11,12; however, they are typically engaged in solution-based catalysis which necessitates the transfer of electrons by catalyst molecules shuttling between the electrode and the bulk solution, constraining catalytic performance13,14,15. Immobilization of functional complexes by anchoring onto conductive substrates such as carbon nanomaterials enables heterogeneous catalysis but they suffer from unsatisfactory stability due to the weak interaction between molecules and substrates16,17,18.
Single-atom catalysts (SAC) inherit the single-site nature of molecular complexes and can be stabilized in inorganic matrices to improve durability19,20. Tuning the electronic state of active sites in SACs is critical in tuning the catalytic performance for its imperative influence on the adsorption of reactants/intermediates on metal atoms21,22. Such tuning is attainable via manipulating chemical environment around the active centers, such as heteroatom doping23, axial coordination24, and secondary coordination modification25. Numerous SAC materials have been reported hitherto; nevertheless, the host matrices have largely been limited to nitrogen-doped carbon, metal oxides, or MXenes26, and homogeneity of SAC active sites is inclined to lose under unavoidable harsh synthesis conditions27,28.
We took the view that a greater degree of freedom in heterogenized single-site catalyst design could be achieved via bottom-up synthetic chemistry at benign conditions29. In our prior report, we discovered a heterogenized molecular catalysts with uniform single-site Cu that was able to catalyse CO2RR stably and selectively30. The catalysts share similar tunability in structures and properties to reticular 3D materials31. This molecular platform allows for rational design of single-site catalysts with accurate control over active sites at an atomic level and, therefore, building the structure-function relationship by systematic tuning of the electron states in the systems32.
Herein, we report a series of Cu-based coordination polymer catalysts that were synthesised via coordination of cupric ion with a family of phenyl-1H-1,2,3-triazole (PhTA) derivatives. These PhTA ligands present a wide range of highest occupied molecular orbital (HOMO) energy, enabling us to tune the electronic structure of catalytically active Cu sites in the formed coordination polymers. Operando X-ray absorption spectroscopy (XAS) and in situ infrared (IR) spectroscopy support that there is no bulk material change under applied potentials relevant to CO2RR. We found a volcano-shape correlation between the HOMO energy of PhTA ligands and the C–C coupling efficiency (ηC–C coupling), a parameter we develop to evaluate the efficiency of C2 production. Using XAS, ultraviolet and visible (UV-vis) spectroscopy, CO diffuse reflectance Fourier transform IR spectroscopy (CO-DRIFTS), in situ Raman spectroscopy and theoretical calculations, we found that the tuning of HOMO energy altered the electronic state of Cu and its binding energy of *CO intermediate, the key intermediate determining C2 selectivity, resulting in the control over ηC–C coupling from 0.26 to 0.86.
Results
Tuneable Cu electronic states by ligand coordination
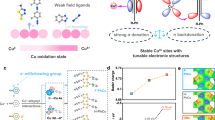
We took inspiration from organometallic chemistry that the propensity of electron transfer from ligand to metal is dominated by the HOMO energy of ligands in coordination compounds with the same metal centers. Specifically, elevation of ligand’s HOMO energy decreases the energy difference between ligands and metal centers, leading to the formation of stronger coordination bonds (Fig. 1a)33. Triazole derivatives, such as PhTA, have been used in anticorrosion as a protective layer on copper surfaces due to the strong chelation with cupric ions via Cu–N bonds34,35. We posited a high stability of the complexes formed via coordination between PhTA and Cu.
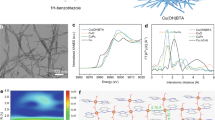
a Schematic diagram of orbital energy level when forming the Cu–N coordination bonds. b Chemical structure of ligand molecules modelled. c HOMO energy of ligand. The anionic form of ligands rather than neutral form was used for the calculation due to deprotonation. d The Cu–N coordination models in the modelling. L1 was illustrated here and L2–L6 was presented in Supplementary Fig. 1. The orange, blue, grey, cyan, and white balls represent Cu, N, C, F, and H, respectively. e The relationship between Bader charge of Cu in Ln-Cu and HOMO energy of the ligands. Source data are provided as a Source data file.
Our calculations showed a wide range of HOMO energy from –5.82 to –5.19 eV of PhTA derivatives by changing the electron donating or withdrawing substituent in the benzene ring (L1–L6, Fig. 1b, c), offering the potential to tune the coordination environment around copper centers. The electronic structure of Cu in the formed Cu–N coordination was modelled based on a simplified coordination structure (Fig. 1d and Supplementary Fig. 1) and the Bader charge of Cu was used as an indicator to assess these derivatives’ tunability of Cu’s electronic state. A linear correlation between Cu Bader charge and ligand’s HOMO energy was identified (Fig. 1e): the electron donating ability of ligands increases along with the HOMO energy, leading to lowered Bader charges on Cu.
Synthesis and characterization of catalysts
Experimentally, we used a one-step click reaction—Cu(I)-Catalysed Azide–Alkyne Cycloaddition (CuAAC)—to synthesise L1–L6 (Fig. 2a, see details in ‘Methods’)36. The products were purified by column chromatography method and the chemical structures were confirmed by 1H nuclear magnetic resonance (NMR) and 13C NMR (Supplementary Information). Coordination reaction of the ligands and CuCl2 in an alkaline solution (pH = 13) led to the formation of coordination polymers Ln-Cu (n = 1–6). In the coordination, the N–H was deprotonated due to the highly alkaline environment (pKa of L2 = 7.68)37. The resulting Ln-Cu have similar crystal packing (X-ray diffraction (XRD), Supplementary Figs. 2–3) and exhibit similar nanowire morphology with a width of 20 ± 10 nm (transmission electron microscopy (TEM), Fig. 2b and Supplementary Fig. 4). Scanning electron microscopy energy-dispersive X-ray spectroscopy (SEM-EDS) mapping shows that N, Cu, and O disperse uniformly (Supplementary Fig. 5).
a Synthetic pathways of ligands and coordination polymers. b TEM image of L2-Cu. c Cu K-edge XANES spectrum of L2-Cu. The inset displays a magnification of the pre-edge peak. d Cu K-edge FT-EXAFS spectrum and fitting of L2-Cu. The k-range of 2.5–11 Å–1 and the R-range of 0–4 Å were used for the fit. The assignment of N/O/C back-scatterers is subjective, and the interatomic distances are based on the structure simulated by DFT. The Cu K-edge FT-EXAFS spectra of Cu foil and CuPc were used as references. e The structure of Ln-Cu. X represents the substituents and is on the right frame. * indicates the position connecting with triazole rings. Source data are provided as a Source data file.
The local atomic structure of Ln-Cu was studied using synchrotron XAS. The absorption edge position of L2-Cu in X-ray absorption near edge spectrum (XANES) was located near CuO and copper(II) phthalocyanine (CuPc), demonstrating a Cu oxidation of +2 (Fig. 2c). Besides, the XANES of L2-Cu showed the K-pre-edge peak, which is characteristic for Cu2+. These results are consistent with Cu 2p X-ray photoelectron spectroscopy (XPS) and LMM spectra (Supplementary Fig. 6). Fourier-transform extended X-ray absorption fine structure (FT-EXAFS) spectra38 and fitting showed a coordination number (CN) of 4 for Cu in the L2-Cu, including two N atoms and two O atoms (Fig. 2d and Supplementary Table 1). Further, single-site Cu showed the best fitting results in comparison with Cu clusters and no metallic Cu–Cu bond was identified (Supplementary Figs. 7–9 and Supplementary Tables 1–3). It is acknowledged that small number of clusters might exist due to the limit of detection of EXAFS technique39. The fitted Ln-Cu structure agreed well with XPS (Supplementary Fig. 6 and Table 4), inductively coupled plasma-atomic emission spectroscopy (Supplementary Table 5) and elemental analyses (Supplementary Table 6). Collectively, the evidence pointed to a Ln-Cu structure where each Cu center coordinated with two N atoms (N1 and N3, Fig. 1d) from two different PhTA molecules to form molecular chains, which were bridged through –OH groups (Fig. 2e, Supplementary Figs. 10–13). Based on the simulated structure, the calculated refinement matches well with the measured XRD patterns (Supplementary Fig. 14).
CO2RR performance and catalyst stability
CO2RR performance of Ln-Cu was measured in a flow cell using 1 M KOH as the electrolyte. The gas and liquid products were identified and quantified by online gas chromatography (GC) and 1H NMR, respectively. The faradaic efficiency (FE) of all products were shown in Fig. 3a and Supplementary Fig. 15. All the Ln-Cu showed similar trend of FE with applied potentials. For example, FEethylene of L2-Cu increased from –0.77 V (versus reversible hydrogen electrode (RHE); all potentials are referenced to RHE unless otherwise noted) to –0.95 V and reached the maximum of 51% at –0.95 V, while FECO gradually decreased (Fig. 3a). The partial current densities for C2 products increased with the decreased applied potentials and reached the maxima of –386 mA cm–2 at –0.95 V. Compared to \({j}_{{{{{\rm{C}}}}}_{2}}\), \({j}_{{{{{\rm{H}}}}}_{2}}\) and \({j}_{{{{{\rm{C}}}}}_{1}}\) gas did not show significant changes, meaning their production rates were neatly constant under the applied potential range (Fig. 3b). L2-Cu showed promising CO2 electrolysis performance using a compact, commercialization-relevant membrane electrode assembly (MEA) system (Supplementary Fig. 16). At a full-cell voltage of 3.6 V, the MEA exhibited a stable current (~600 mA) and ethylene selectivity for an initial test of 50 h (Supplementary Fig. 17). No noticeable change of the post-electrolysis L2-Cu sample was observed via XRD (Supplementary Fig. 18). Additionally, SEM images (Supplementary Fig. 19) in several areas exhibit the well-preserved nanowire morphology. SEM-EDS mapping demonstrates the uniform disperse of Cu after CO2RR (Supplementary Fig. 20 and Table 5), and XPS spectra of Cu 2p, N 1s, O 1s, and F 1s (Supplementary Figs. 21–24) show no change compared to as-prepared Ln-Cu. These results collectively suggest that the bulk materials retained their morphologies and chemical properties after CO2RR.
a CO2RR performance of L2-Cu at applied potentials from −0.77 to −0.95 V. b Partial current densities of H2, C1 gas (CO and CH4), and C2 (C2H4, C2H6O, CH3COO–) products under potentials from −0.77 to −0.95 V. c In situ FTIR spectra of L2-Cu in CO2-saturated 0.1 M KHCO3 solution. The *COatop peaks red-shifted from 2060 cm–1 at −0.3 V to 2014 cm–1 at −1.1 V. When reversing the applied potential back to –0.8 V and –0.7 V, the intensities of *CO recovered and the peaks shifted to 2039 and 2045 cm–1, respectively. d Operando Cu K-edge XANES of L2-Cu at the applied potentials of −0.69 V to −0.79 V during CO2RR in a customised flow cell. Reference samples: Cu (black), Cu2O (yellow), and CuO (grey). The potentials in (c) and (d) are E – iR. Source data are provided as a Source data file.
We further conducted operando/in situ spectroelectrochemical studies to explore the structural stability of Ln-Cu under operating conditions. Using L2-Cu as a representative, we only observed atop adsorbed *CO intermediate (*COatop) formed under applied potentials from –0.3 to –1.1 V in the in situ IR spectra, with peaks shifting from 2060 to 2014 cm–1 (Fig. 3c). In contrast, both *COatop and *CObridge were found using metallic Cu catalysts40,41 or coordination polymer derived Cu catalyst in our prior study30. When reversing the applied potential back to –0.8 V and –0.7 V followed by the measurement at –1.1 V, the *CO peak intensities recovered and the *CO peaks blue-shifted to 2,039 and 2,045 cm–1, respectively, the same positions as those in the initial scans. These results suggested no Cu agglomeration formed to be observable by IR spectroscopy during CO2RR process. The Stark effect of CO vibrational signals confirmed that *CO was adsorbed species42,43,44.
We then explored the nature of Cu local environment by conducting operando XAS of the Cu K-edge in a customized flow cell (Supplementary Figs. 25–26). The XANES spectra showed that the oxidation states of Cu under all applied potentials are close to +2, with slight decrease under applied potential from –0.69 to –0.79 V (Fig. 3d). FT-EXAFS fitting showed that the slight change of Cu oxidation state originated from the dynamic coordination environment during CO2RR process (Supplementary Fig. 27). The fitting of FT-EXAFS spectra showed that total CN of Cu in L2-Cu remained 3.9–4.3 under the potential range of –0.69 to –0.79 V. The CN of Cu–N was ~2.0 and did not change under varied potentials. CN of Cu–O decreased with the increase of CN of Cu–C under applied potentials (Supplementary Figs. 28–29 and Tables 8–10). Besides, the CN of Cu–O bond recovered to ~2 with the disappearance of Cu–C bond when the applied potential was reversed to open circuit potential (OCP) state (Supplementary Table 11). These results indicated the predominant presence of single-site Cu atoms in L2-Cu catalysts under applied potentials, serving as the active sites for binding CO2RR reactants and intermediates.
Substitution of –Cl for –OH as the bridging group in L2-Cu resulted in similar CO2RR performance and the L2-Cu(Cl) became L2-Cu(OH) after the reaction (Supplementary Fig. 30). This solid-to-solid transformation through ligand exchange did not lead to structural reconstruction and implied that –OH was prone to de-coordinate and leave the site vacant for CO2RR.
C–C coupling efficiency
The wide tunability of HOMO energy in the synthesised coordination polymers allowed us to study the structure-performance relationship between the electronic properties of Cu and the CO2RR selectivity. We observed a volcano-type plot for the C2 selectivity of Ln-Cu catalysts and L2-Cu showed a maxima \({{{{\rm{FE}}}}}_{{{{{\rm{C}}}}}_{2}}\) of 77% at –500 mA cm–2 (Fig. 4a). Contact angle measurements illustrate that the difference in the observed results is not correlated to hydrophobicity of the catalysts (Supplementary Fig. 31). As C–C coupling is the most critical step toward C2 production in CO2RR45,46, we developed the C–C coupling efficiency (ηC–C coupling) to assess the probability of *CO to embark on path toward C2. The ηC–C coupling was calculated using the following Eq. (1):
a FE for C1 (CO and CH4) and C2 (C2H4, CH3COO–, C2H6O) on Ln-Cu at the same current density of –500 mA cm–2. b The relationship between FE of C2 and ηC–C coupling of all Ln-Cu catalysts at current densities from –100 mA cm–2 to –500 mA cm–2. c Coloured contour map of ηC–C coupling. d ηC–C coupling of Ln-Cu at –0.930 ± 0.008 V. Source data are provided as a Source data file.
accounting for the three paths for adsorbed *CO: desorption to gaseous CO, deep hydrogenation to CH4, or dimerization to C2 products46.
As shown in Fig. 4b, the ηC–C coupling over all Ln-Cu across the applied potential window was positively correlated with C2 selectivity, suggesting that ηC–C coupling was adequate to assess the dimerization ability of *CO. The coloured contour map showed the ηC–C coupling increasing from −0.76 to −0.96 V in all Ln-Cu samples (Fig. 4c), which prior studies have ascribed to the higher *CO coverage under more negative applied potentials47. At the same applied potential, such as −0.93 V, the ηC–C coupling presented a volcano-shape amongst L1-Cu to L6-Cu and reached the top of 0.81 for L2-Cu (Fig. 4c, d).
Mechanism studies
We sought to understand the reaction mechanism, examining how the electronic structures of Ln would lead to varied ηC–C coupling of Ln-Cu. In the simplified model, we have confirmed that the HOMO energy of ligand was related to the Bader charge of chelated Cu (Fig. 1e). To verify such correlation in the synthesised coordination polymers, solid UV-vis absorption spectra were collected (Fig. 5a). The red shift of absorption wavelength from L1-Cu to L6-Cu implied increasingly enhanced electron donating capacity of Ln to the Cu center48. Correspondingly, a decrease of Cu oxidation state from L1-Cu to L6-Cu was evident from the red shift of absorption edge in the Cu K-edge XANES (Fig. 5b). The absorption wavelength of an organometallic complex is correlated to the energy difference between HOMO energy of ligand and low unoccupied molecular orbital (LUMO) energy of metal centers. As all the Ln-Cu materials have the same Cu center, the energy difference is determined by the HOMO energy of ligands. A linear correlation of the HOMO energy with the shift of absorption wavelength was found, in concordance with that between the HOMO energy and the XANES edge shift (Fig. 5c). This indicated that the enhanced covalent interactions between Cu2+ and Ln ligands led to lowered oxidation state of Cu in the formed Ln-Cu.
a Solid UV-vis absorption spectra of Ln-Cu. b Cu K-edge XANES spectrum of Ln-Cu. c The linear relationship between HOMO energy of ligands and UV-vis absorption wavelength along with XANES energy shift for Ln-Cu. The wavelength is the peak value of each absorption spectrum. XANES energy shift indicates the absorption energy difference compared to that of L6-Cu. d CO decay curves which shows the desorption rate of adsorbed CO on Cu active sites in different catalysts. e In situ Raman spectra of Ln-Cu at E – iR = –0.86 V. f The relationship between *CO binding strength and ηC−C coupling based on the trend of HOMO energy. The time at normalised intensity = 0.5 is denoted as t0.5, which was calculated from Fig. 5d based on the exponential equation (see details in ‘Methods’); Raman shift of *CO was picked up from the peak value of Raman spectra (shaded in blue) shown in Fig. 5e. Source data are provided as a Source data file.
In previous studies, the adsorption of *CO intermediate plays a critical role in determining ηC–C coupling46,49,50. We therefore further studied the *CO binding strength on Cu in Ln-Cu catalysts. CO-DRIFTS was firstly used to study the desorption rate of adsorbed CO (Supplementary Fig. 32). All absorption intensities of the CO desorption process were normalised and fitted by an exponential function (see details in ‘Methods’) and showed a decreased desorption rate from L1-Cu to L6-Cu (Fig. 5d), suggesting an increase of CO binding strength from L1-Cu to L6-Cu.
In situ Raman spectroscopy was then conducted to examine the adsorption behaviours of *CO generated in situ during CO2RR turnovers (Supplementary Figs. 33–34)51. For each Ln-Cu catalyst, the C≡O stretch in the range of 1900–2200 cm−1 also showed the Stark effect, shifting to lower wavenumbers with applied potentials from –0.10 to –1.01 V. The intensity of *CO peaks initially increased followed by a slight decrease, which prior report ascribed to the rapid consumption of *CO intermediates under more negative potentials52. To compare the binding strength of *CO between Ln-Cu catalysts, the Raman spectra at –0.86 V were summarised in Fig. 5e. The *COatop stretch shifted from 2067 cm–1 in L1-Cu to 2036 cm–1 in L6-Cu. This redshift has been attributed to a stronger binding of CO to the Cu site53. The interference by vibrational signals of PhTA ligands prevented analysis of Cu–CO stretch located at about 370 cm−1, another Raman shift related to binding strength of *CO52. Besides, the Raman peaks from 250 to 1650 cm−1, positions for typical vibrational modes of Ln-Cu coordination polymers, have been assigned in Supplementary Fig. 35. The spectra obtained during and after the reaction indicated the retention of ligands on the electrode surface (Supplementary Figs. 36–42). The *CO binding strength obtained from both CO-DRIFTS and in situ Raman spectroscopy was found to be linearly correlated to the HOMO energy of Ln (Fig. 5f). This correlation was further supported by the calculated CO adsorption energies on Cu active sites of Ln-Cu using density functional theory (DFT, Supplementary Fig. 43).
Comparing Fig. 5c and f, it was evident that the HOMO energy of PhTA derivatives in the coordination polymers was able to modulate the electronic states of chelated Cu, which resulted in the tuning of Cu’s binding strength toward *CO. High electron-donating ability of the ligand decreased the oxidation state of Cu. *CO bonds with Cu (orbital structure Cu(2-δ)+ ([Ar]3d9+δ)) through its 5σ orbital and π* orbital (i.e., back-donation) and the contribution share of each determines the overall binding strength54. The moderate Cu oxidation state in L2-Cu showed appropriate CO binding energy, the key to lowering *CO dimerization and enabling an optimal ηC−C coupling (Fig. 5f)55. The resulting CO dimer showed an energetically preferable adsorption on two neighbouring Cu sites, where the Cu–Cu distance of 3.57 Å matched well with that in the pristine L2-Cu coordination structure (3.38–3.44 Å, Supplementary Fig. 44). The trivial change of Cu–Cu distance assists in the structural stability during CO2RR.
Discussion
We designed and synthesised a series of structurally stable single-site catalysts Ln-Cu for electrochemical CO2 reduction. Tuning the electron states of Cu in these Ln-Cu catalysts was achieved by leveraging a wide range of HOMO energies present in the ligands, consequently influencing the adsorption energy of *CO intermediates. Using in situ Raman and CO-DRIFTS analysis, we established a volcano-shaped correlation between the binding strength of *CO on the homogenized Cu active sites and the ηC−C coupling. Further studies are warranted to synthesise and investigate similar coordination polymers with wider tunability of electronic properties and reaction intermediate adsorption energies, allowing for further structure-function correlations and devising better-performing catalysts.
Methods
Chemicals and materials
Copper(II) chloride dihydrate (CuCl2·2H2O, AR), sodium hydroxide (NaOH, AR), potassium bicarbonate (KHCO3, AR), potassium hydroxide (KOH, AR), sodium sulfate (Na2SO4, anhydrous, AR), hydrochloric acid (HCl, CP, 36–38%), nitric acid (HNO3, AR, 65.0–68.0%), methanol (MeOH), ethanol, N,N-Dimethylformamide (DMF), ethyl acetate (EtOAc), isopropanol, and acetone were all purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). 4-Phenyl-1H-1, 2, 3-triazole (PhTA) (C8H7N3) was purchased from Accela Chembio. Azidotrimethylsilane (TMSN3), cuprous iodide (CuI), 2-fluorophenylacetylene, 4-fluorophenylacetylene, 4-chlorophenylacetylene, 4-methylphenylacetylene, and 4-methoxyphenylacetylene were purchased from Bide Pharmatech Ltd. (Shanghai, China). Copper(II) phthalocyanine (CuPc), 3-(trimethylsilyl)−1-propanesulfonic acid sodium salt (DSS), (dimethyl sulfoxide)-d6 (DMSO-d6), and Nafion solution (~5 wt%) were purchased from Sigma-Aldrich. Proton exchange membrane (Nafion 115) was purchased from Dupont. Anion exchange membrane (Sustainion x37-50-grade-60) was purchased from Dioxide Materials. Titanium gauze (100 mesh) and iridium(III) chloride hydrate (IrCl3·3H2O) were obtained from Alfa Aesar. Carbon paper-based gas diffusion layers (GDLs, Sigracet 29 BC) were purchased from the Fuel Cell Store. Ultrapure Millipore water (resistivity 18.2 MΩ cm) was used for all experiments. All the chemicals were used without further purification.
Synthesis of triazole derivatives (ligands Ln)
An oven-dried vial (100 mL) was equipped with a magnetic stir bar and charged with CuI (95 mg, 0.5 mmol). The vial was sealed with a screw-cap septum, and evacuated and backfilled with argon. Then, the solvent of DMF/MeOH (9:1, 20 mL) was added via syringe. TMSN3 (2.0 mL, 15 mmol) and phenylacetylene derivatives (10 mmol) were then added to the reaction vial subsequently. The resulting mixture was stirred at 100 °C for 12 h. After the reaction was finished, water was added and the reaction mixture was extracted with EtOAc (25 mL × 3), dried over Na2SO4, and concentrated in vacuo. The crude mixture was purified by flash column chromatography (petroleum ether/ethyl acetate = 10/1 to 2/1) to give corresponding products. The characterization data of products can be found in Supplementary Information.
Synthesis of Ln-Cu catalysts
CuCl2·2H2O (85.25 mg, 0.5 mmol) was added into 20 mL of ultrapure water to obtain a blue aqueous solution. Ligand Ln (1 mmol), NaOH (0.24 g, 6 mmol), and 40 mL of ultrapure water were mixed to obtain a colourless clear solution. The CuCl2 aqueous solution was quickly pulled into the mixture, and then stirred at room temperature for 11 h. After that, the obtained blue colloid was centrifuged and washed with deionized H2O for three times. Finally, the sediments were dried at 45 °C overnight under vacuum. The as-synthesised sample was used as the catalyst (denoted as Ln-Cu) after grinding.
Preparation of working electrodes
15 mg of the catalyst powder was dispersed in 2 mL of methanol and sonicated to get an evenly dispersed mixture. Then, 60 µL of a Nafion solution (~5 wt%) was added, followed by additional sonication to produce the catalyst ink. The ink was spray-coated onto a 2 × 4 cm2 GDL to fabricate gas diffusion electrodes (GDEs), achieving a catalyst loading amount of ~0.8 mg cm–2. The prepared electrodes were dried under ambient conditions overnight. This same method was also used to prepared electrodes for the in situ Raman and operando XAS measurement.
Preparation of the anode IrO2/Ti mesh
The anode was prepared using dip-coating followed by thermal decomposition56. In detail, 30 mg of IrCl3·3H2O was dissolved in a mixture of 9 mL of isopropanol and 1 mL of 6 M HCl at 80 °C to firstly prepare the dip-coating solution. A 2 cm × 2 cm Ti mesh was cleaned with water and acetone using ultrasound to remove oil stains, followed by etching in 6 M HCl for 30 min. The etched Ti mesh was then immersed in the dip-coating solution and dried under infrared light. The dried Ti mesh was calcined in a furnace at 500 °C for 10 minutes, and the calcined Ti mesh was weighed to determine the loading amount of the catalyst. This dip-coating, drying, and calcining process was repeated several times until the loading amount of IrO2 on Ti mesh reached approximately 2.0 mg cm–2.
Electrochemical measurements
CO2RR performance tests were performed in a flow cell using a three-electrode system. A Ag/AgCl electrode (saturated KCl solution) served as the reference electrode, a Pt wire acted as the counter electrode, and Ln-Cu catalysts on carbon paper were used as the working electrodes (area: 1 cm2). Fresh 1 M KOH, prepared using a volumetric flask, was used as both the catholyte and anolyte. The electrolyte was circulated through the flow cell at a constant rate of 5 mL min–1 using a peristaltic pump. The proton exchange membrane (Nafion 115) was used as the ion exchange membrane. Stability tests were performed in the MEA system using a two-electrode system. IrO2/Ti mesh was used as the anode, and L2-Cu on carbon paper was used as cathode (area: 4 cm2). Anion exchange membrane (Sustainion x37-50-grade-60) was used as the ion exchange membrane. The anolyte was 0.1 M KHCO3. The gaseous 95% CO2 (5% N2 as internal standard) was flowed through the gas chamber. The flow rate was monitored by a mass flow controller at a rate of ~20 standard cubic centimeters per minute (sccm). The gas was humidified before being supplied to the MEA system. The flow rate of the electrolyser outlet stream was measured by soap bubble flow meter. All the electrocatalytic reactions were conducted at ambient pressure and temperature. The electrochemical workstation was CHI 1140C.
All potentials vs the Ag/AgCl electrode were converted to the potentials vs RHE based on the following Eq. (2):
The applied potentials were compensated with solution resistance and noted as E – iR, where R is the ohmic resistance between the working and the reference electrode, which was defined using electrochemical impedance spectroscopy analysis prior to the measurement (ohm), and i is current (A). The measured R at OCP state was about 5 ohm for all Ln-Cu catalysts at the flow cell tests. The non-iR corrected voltammograms for all catalysts studied were supplied in Supplementary Fig. 15f. The pH of the electrolyte was adjusted to 14 using a pH meter.
Quantification of products
Gas-phase products were measured by gas chromatography (GC2014, Shimadzu, Japan). 5% N2 contained in feed gas was used as an internal standard to quantify the gas products. The FE of the gas products can be obtained according to the peak area of the specific products.
Liquid products were measured using 400 MHz 1H-NMR. DMSO-d6 severed as the lock solvent. DSS serves as an internal standard. The concentration of liquid products was quantified by comparing the area ratio of the liquid product peaks to the DSS peak against a standard curve (Supplementary Fig. 45).
The FE for a specific product was calculated using the following Eq. (3):
Where:
Cproduct is the measured concentration of the specific product (mol/L);
V is the volume of the electrolyte for liquid products or the total gaseous volume for gaseous products (L);
N is the number of electrons transferred for the formation of the specific product;
F is Faradaic constant (96,485 C mol–1);
Q is the total quantity of electric charge (C).
Characterization
The morphologies of samples were recorded by SEM (Zeiss Sigma VP 3view). SEM-EDS mapping was mapped by Zeiss Sigma VP HD. TEM images were acquired using a Talos F200X field-emission transmission electron microscope operating at an accelerating voltage of 200 kV. To prepare the sample for TEM imaging, 0.1 mg of Ln-Cu samples were mixed with 0.5 mL of ethanol solution and sonicated for 1 hour. The resulting evenly-dispersed solution was drop-casted onto a Lacey Carbon Supported Mo grid (200 mesh) and dried under infrared light. For XRD measurement, each powder sample was loaded into the 0.2 mm deep cavity of a glass sample holder. XRD data from the spinning samples were collected in Bragg-Brentano geometry on a Rigaku Smartlab SE powder diffractometer equipped with a Cu Kα X-ray source (Kα1 = 1.540598 Å, Kα2 = 1.544426 Å) operating at 40 kV and 40 mA, a 10 mm length-limiting slit and ½° divergence slits. Diffraction data were collected using an XSPA-400 ER two-dimensional detector. The transmitted infrared spectra were carried out by a FT-IR spectrometer (Thermo Scientific Nicolet is50). The Raman spectra were recorded by Lab RAM HR Evolution (Horiba). The XPS spectra were recorded on a Thermo ESCALAB250Xi spectrometer with an excitation source of monochromatized Al Kα (hν = 1486.6 eV) and a pass energy of 40 eV. The values of binding energies were calibrated with the C 1s peak of contaminant carbon at 284.80 eV. UV-vis spectra of the catalysts were acquired on the Shimazu UV-2700.
CO-DRIFTS measurements
CO-DRIFTS measurements were performed in an elevated-pressure cell (DiffusIR Accessory PN041-10XX) using a Thermo Scientific Nicolet iS50 FTIR spectrometer. Firstly, Ar gas was flowed through the cell at 1 bar and a rate of 30 sccm at 100 °C for 30 min, followed by cooling to 25 °C to acquire a flat line as the background spectrum. Next, 20% CO in Ar at 1 bar was purged into the cell at a rate of 20 sccm at 25 °C until the absorbance of CO was saturated. Finally, Ar was purged at 1 bar and a rate of 30 sccm at 25 °C, and the spectra of this process were recorded to generate the decay curves.
For any given catalyst, all absorption intensities from 0 to 600 s were normalised by the initial absorption intensity at t = 0. We then used the ExpAssocDecay1 function (Eq. (4)) for curve fitting using OriginPro:
TD (Time offset): x value at which exponential begins;
Yb (Baseline): y value before exponential begins;
A (Amplitude): change in response;
Tau: Time constant
In situ Raman measurements
In situ Raman was carried out using Lab RAM HR Evolution (Horiba) equipped with an Olympus x60 water immersion lens (NA 1.0, WD 2.0 mm) in a home-made flow cell with a 785 nm laser with 50% intensity. 0.5 M KOH solution was used as supporting electrolyte. An Ag/AgCl (saturated KCl) electrode and a Pt wire were used as the reference and counter electrodes, respectively. CO2RR products were not analysed during in situ Raman test. Each spectrum was collected by integration twice, 10 s per integration. One sets of spectra were collected under CO2 with a flow rate of 5 sccm.
In situ IR measurements
In situ FTIR was carried out using a Thermo Scientific Nicolet iS50 FTIR spectrometer with internal reflection configuration at room temperature. An Au film was first chemically deposited on a smooth surface of a Si prism. 4 mg catalysts were mixed with 0.5 mL iso-propanol and 8 μL Nafion solution to make an ink which was further sprayed onto the cover of the Au film. CO2-saturated 0.1 M KHCO3 was used as the electrolyte in the measurements. CO2RR products were not analysed during in situ IR test. All spectra were collected in absorbance mode at a resolution of 4 cm−1 while linear sweep voltammetry was conducted at the same time with a scan rate of 2 mV s−1.
XAS measurements
All XAS spectra were collected at the Australian Synchrotron in Clayton, Victoria, Australia, using the high-flux 1.9 T wiggler XAS Beamline operating in slow scan (continuous energy) mode. Ex situ XAS spectra were collected in transmission mode on sample pellets prepared as a homogenous mixture of catalyst powder with cellulose as a binding and filling agent. Operando XAS measurements on prepared catalysts were conducted in fluorescence mode in a home-made flow cell with a window sealed by Kapton tape (Supplementary Fig. 26). Similar CO2RR product distribution and selectivity were obtained using the operando XAS cell, compared to those using the flow cell for CO2RR performance test57,58.
XAFS analysis
EXAFS raw data were processed with the Demeter (v.0.9.26) software package59. The Athena software was employed to conduct energy calibration (using a Cu foil standard), background subtraction, and spectral normalisation. Least-squares curves fitting analysis of the EXAFS χ(k) data was carried out across the range of 2.5–11 Å−1 using the Artemis software.
Computational methods
All the density functional theory (DFT) were carried out by the Vienna ab initio simulation program (VASP)60,61. The generalized gradient approximation (GGA) with Perdew-Burke-Ernzerhof (PBE) was used to describe the exchange-correlation term62. The projector-augment wave methods was adopted to describe the electron-ion interactions63. The cut-off energy was set as 450 eV. The inter-molecule interactions was taken into consideration by D3 correction method proposed by Grimme64. The implicit solvation model implemented in VASPsol was used to consider the effect of solvent65.
Data availability
All data generated in this study are provided in the Supplementary Information/Source data file. Source data are provided with this paper.
Change history
09 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-024-55732-7
References
Badwal, S. P., Giddey, S. S., Munnings, C., Bhatt, A. I. & Hollenkamp, A. F. Emerging electrochemical energy conversion and storage technologies. Front Chem. 2, 79 (2014).
Brinkert, K. & Mandin, P. Fundamentals and future applications of electrochemical energy conversion in space. npj Microgravity 8, 52 (2022).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Chen, X. et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 4, 20–27 (2020).
Choi, C. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 3, 804–812 (2020).
Stephens, I. E. L. et al. 2022 Roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022).
Bouckaert, S. et al. Net Zero by 2050 (International Energy Agency, 2021).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050 (2012).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Woldu, A. R., Huang, Z., Zhao, P., Hu, L. & Astruc, D. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord. Chem. Rev. 454, 214340 (2022).
Zhu, H. L. et al. A porous pi-pi stacking framework with dicopper(I) sites and adjacent proton relays for electroreduction of CO2 to C2+ products. J. Am. Chem. Soc. 144, 13319–13326 (2022).
Ambre, R. B. et al. Molecular engineering for efficient and selective iron porphyrin catalysts for electrochemical reduction of CO2 to CO. Chem. Commun. 52, 14478–14481 (2016).
Han, J. et al. Bioinspired iron porphyrins with appended poly-pyridine/amine units for boosted electrocatalytic CO2 reduction reaction. eScience 2, 623–631 (2022).
Zheng, J. et al. Non-negligible axial ligand effect on electrocatalytic CO2 reduction with iron porphyrin complexes. J. Phys. Chem. Lett. 13, 11811–11817 (2022).
Francke, R., Schille, B. & Roemelt, M. Homogeneously catalyzed electroreduction of carbon dioxide-Methods, mechanisms, and catalysts. Chem. Rev. 118, 4631–4701 (2018).
Oh, S., Gallagher, J. R., Miller, J. T. & Surendranath, Y. Graphite-conjugated rhenium catalysts for carbon dioxide reduction. J. Am. Chem. Soc. 138, 1820–1823 (2016).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Zhang, X. et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat. Energy 5, 684–692 (2020).
Sui, J. et al. A general strategy to immobilize single-atom catalysts in metal-organic frameworks for enhanced photocatalysis. Adv. Mater. 34, e2109203 (2022).
Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819–7827 (2021).
Cai, Y. et al. Insights on forming N,O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat. Commun. 12, 586 (2021).
Cao, D. et al. Volcano-type relationship between oxidation states and catalytic activity of single-atom catalysts towards hydrogen evolution. Nat. Commun. 13, 5843 (2022).
Yang, H. B. et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Zhang, T. et al. Pinpointing the axial ligand effect on platinum single-atom-catalyst towards efficient alkaline hydrogen evolution reaction. Nat. Commun. 13, 6875 (2022).
Pan, F. et al. Boosting CO2 reduction on Fe-N-C with sulfur incorporation: synergistic electronic and structural engineering. Nano Energy 68, 104384 (2020).
Lai, W. H., Miao, Z., Wang, Y. X., Wang, J. Z. & Chou, S. L. Atomic-local environments of single-atom catalysts: Synthesis, electronic structure, and activity. Adv. Energy Mater. 9, 1900722 (2019).
Bai, X. et al. Dynamic stability of copper single-atom catalysts under working conditions. J. Am. Chem. Soc. 144, 17140–17148 (2022).
Yang, J. et al. Potential-driven restructuring of Cu single atoms to nanoparticles for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 144, 12062–12071 (2022).
Zhang, T. et al. Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion. Nat. Commun. 12, 5265 (2021).
Liang, Y. et al. Stabilizing copper sites in coordination polymers toward efficient electrochemical C-C coupling. Nat. Commun. 14, 474 (2023).
Franco, F., Rettenmaier, C., Jeon, H. S. & Roldan Cuenya, B. Transition metal-based catalysts for the electrochemical CO2 reduction: from atoms and molecules to nanostructured materials. Chem. Soc. Rev. 49, 6884–6946 (2020).
Wu, H. et al. Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization. Nat. Energy 9, 422–433 (2024).
Di Girolamo, A. et al. 4-Phenyl-1,2,3-triazoles as versatile ligands for cationic cyclometalated iridium(III) complexes. Inorg. Chem. 61, 8509–8520 (2022).
Li, W. et al. Insights into triazole derivatives as potential corrosion inhibitors in CMP process: experimental evaluation and theoretical analysis. Appl. Surf. Sci. 602, 154165 (2022).
Finšgar, M. & Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: a review. Corros. Sci. 52, 2737–2749 (2010).
Meldal, M. & Diness, F. Recent fascinating aspects of the CuAAC click reaction. Trends Chem. 2, 569–584 (2020).
Wamhoff, H. 4.11 - 1,2,3-Triazoles and their benzo derivatives. Compr. Heterocycl. Chem. 5, 669–732 (1984).
Boubnov, A., Gremminger, A., Casapu, M., Deutschmann, O. & Grunwaldt, J.-D. Dynamics of the reversible inhibition during methane oxidation on bimetallic Pd-Pt catalysts studied by modulation-excitation XAS and DRIFTS. ChemCatChem 14, e202200573 (2022).
Finzel, J. et al. Limits of detection for EXAFS characterization of heterogeneous single-atom catalysts. ACS Catal. 13, 6462–6473 (2023).
Zhu, S., Jiang, B., Cai, W. B. & Shao, M. Direct observation on reaction intermediates and the role of bicarbonate anions in CO2 electrochemical reduction reaction on Cu surfaces. J. Am. Chem. Soc. 139, 15664–15667 (2017).
Gunathunge, C. M., Ovalle, V. J., Li, Y., Janik, M. J. & Waegele, M. M. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 8, 7507–7516 (2018).
Wei, X. et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 10, 4103–4111 (2020).
Kim, Y. et al. Time-resolved observation of C–C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction. Energy Environ. Sci. 13, 4301–4311 (2020).
Chang, X. et al. Determining intrinsic stark tuning rates of adsorbed CO on copper surfaces. Catal. Sci. Technol. 11, 6825–6831 (2021).
Garza, A. J., Bell, A. T. & Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 8, 1490–1499 (2018).
Todorova, T. K., Schreiber, M. W. & Fontecave, M. Mechanistic understanding of CO2 reduction reaction (CO2RR) toward multicarbon products by heterogeneous copper-based catalysts. ACS Catal. 10, 1754–1768 (2019).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Li, Y. et al. Influence of electron-donating ability of ligand and pH value on MLCT properties of cyanido-bridged complexes. Inorg. Chem. Commun. 140, 109446 (2022).
Handoko, A. D., Wei, F., Jenndy, Yeo, B. S. & Seh, Z. W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 1, 922–934 (2018).
Kuo, T.-C. et al. First-principles study of C–C coupling pathways for CO2 electrochemical reduction catalyzed by Cu(110). J. Phys. Chem. C. 125, 2464–2476 (2021).
Cao, Y. et al. Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions. Nat. Commun. 14, 2387 (2023).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Fielicke, A., Gruene, P., Meijer, G. & Rayner, D. M. The adsorption of CO on transition metal clusters: a case study of cluster surface chemistry. Surf. Sci. 603, 1427–1433 (2009).
Sung, S. S. & Hoffmann, R. How carbon monoxide bonds to metal surfaces. J. Am. Chem. Soc. 107, 578–584 (1985).
Yang, W. et al. Why is C–C coupling in CO2 reduction still difficult on dual-atom electrocatalysts? ACS Catal. 13, 9695–9705 (2023).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Richard, D. et al. Quantifying transport and electrocatalytic reaction processes in a gastight rotating cylinder electrode reactor via integration of Computational Fluid Dynamics modeling and experiments. Electrochim. Acta 440, 141698 (2023).
Shen, K. et al. On the origin of carbon sources in the electrochemical upgrade of CO2 from carbon capture solutions. Joule 7, 1260–1276 (2023).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens Matter Mater. Phys. 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Acknowledgements
F.L. is grateful to his Australian Research Council Discovery Early Career Researcher Award (project number DE200100477) and the ARC Centre of Excellence for Green Electrochemical Transformation of Carbon Dioxide (CE230100017), funded by the Australian Government. J.Z. and F.L. are grateful to the International Partnership Program of Chinese Academy of Sciences (123GJHZ2022101GC) funded by the Chinese Academy of Sciences. J.Z. acknowledges National Key Research and Development Program of China (2021YFA1500500, 2019YFA0405600), CAS Project for Young Scientists in Basic Research (YSBR-051), National Science Fund for Distinguished Young Scholars (21925204), NSFC (U19A2015, 22221003, 22250007), Fundamental Research Funds for the Central Universities, K. C. Wong Education (GJTD-2020-15), Collaborative Innovation Program of Hefei Science Center, CAS (2022HSC-CIP004), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (YLU-DNL Fund 2022012). The computational study is supported by the Marsden Fund Council from Government funding (21-UOA-237) and Catalyst: Seeding General Grant (22-UOA-031-CGS), managed by Royal Society Te Apārangi. All DFT calculations were carried out on the New Zealand eScience Infrastructure (NeSI) high-performance computing facilities. P.W. acknowledges support from Youth Innovation Promotion Association CAS (2020448). B.J. is supported by a Fellowship at the University of Wollongong. Part of this research was undertaken on the XAS beamline at the Australian Synchrotron (17422, 18762, 20139), part of ANSTO, and at the USTC Center for Micro and Nanoscale Research and Fabrication. The authors thank the staff of beamline BL13SSW at Shanghai Synchrotron Radiation Facility for experiments support. This research was supported in part by an AINSE Ltd. Early Career Researcher Grant (ECRG) and the grand challenges program ‘Solar Fuels’ by The University of Sydney Nano Institute.
Author information
Authors and Affiliations
Contributions
F.L., J.Z., P.W. and Z.W. supervised this project. Y.Y. performed most of the CO2RR experiments and analysed the experimental data. Cheng Z. synthesised the ligands. Chengyi Z. carried out DFT calculations and analysed the computational data. Y.S. and L.Z. provided help in measuring CO2RR performances. J.L. analysed the XAS data. Qiang S., S.Z., H.Z., B.J. and J.Z. conducted XAS measurement. J.H. conducted SEM imaging and SEM-EDS mapping. Y.L., Y.S. and Qingqing S. helped in measuring the in situ Raman spectra. J.K., Y.Z. and Z.G. helped in data analysis and organisation. Y.Y. and F.L. wrote and revised the manuscript. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alexey Boubnov, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Zhang, C., Zhang, C. et al. Ligand-tuning copper in coordination polymers for efficient electrochemical C–C coupling. Nat Commun 15, 6316 (2024). https://doi.org/10.1038/s41467-024-50791-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50791-2
This article is cited by
-
The promises and reality of metal–CO2 batteries
Nature Reviews Clean Technology (2025)