Abstract
Transparent, inorganic composite materials are of broad interest, from structural components in astronomical telescopes and mirror supports to solid-state lasers, smart window devices, and gravitational wave detectors. Despite great progress in material synthesis, it remains a standing challenge to fabricate such transparent glass composites with high crystallinity (HC-TGC). Here, we demonstrate the co-solidification of a mixture of melts with a stark contrast in crystallization habit as an approach for preparing HC-TGC materials. The melts used in this approach are selected so that glass formation and crystal precipitation occur simultaneously and synergistically, avoiding the formation of interfacial cracks, residual pores, and delamination effects. Using this method, various unusual hybridized HC-TGC materials such as oxychloride, oxybromide, and oxyiodide composite systems were fabricated in dense, bulk shapes. These materials exhibit intriguing optical properties and neutron response-ability. Using such HC-TGC materials, we develop a neutron detector and demonstrate the application for efficient neutron monitoring and even single neutron detection. We expect that these findings may help to bring about a generation of fully inorganic, transparent composites with synergistic combinations of conventionally incompatible materials.
Similar content being viewed by others
Introduction
The rational combination of inorganic glass and crystal phases into transparent, fully inorganic composite materials is of great interest in fields where the processing ability and surface quality of dense, bulk glasses can be combined with the optical, electronic, or magnetic functionality of the crystalline phase. Such materials have found applications in fields ranging from optical memory1,2, large-capacity telecommunication3,4, smart window5,6, and advanced lighting7,8,9,10,11 to gravitational wave detection12,13. Although recent progress in the synthesis of transparent glass composites (TGCs) has led to a rich library of materials, a remaining challenge has been the fabrication of TGCs with exceptionally high crystallinity (HC-TGC)14. Attempts to overcome this bottleneck include high-pressure assisted sintering or crystallization, containerless15,16 or arc-image processing17, and glass ceramic processing through direct crystallization of a precursor glass18. All of these approaches underly stringent experimental limitations. For example, a fundamental prerequisite of optical transparency is a sufficiently low crystallite size, optical isotropy, and reduced variations in optical refraction between the composite partners and at their phase boundaries. The selection of glass chemical formulations known to exhibit spatially homogeneous, controllable bulk crystallization to a volume fraction beyond 80% is still small. In particular, thermally incompatible materials with strongly deviating stability ranges, or materials with high mutual solubility are challenging to combine within a single composite. Moreover, accurate microstructural design is usually possible only within narrow processing windows, further limited by secondary crystallization effects, phase boundary reactions, and crystal growth19,20,21. For these reasons, most current TGCs derive from oxide phases; combining non-oxide crystals with an oxide glass matrix at a high crystal volume fraction has been outside of current technology.
Neutron detection is widely used in various scientific and technological fields, such as high-energy physics, geological resource exploration, and nuclear medicine22,23,24,25,26. Since neutrons do not interact with matter through Coulomb forces, it has been challenging to realize neutron detection. Currently employed methods, therefore, employ indirect detection using nuclear reactions. Such methods rely on elemental isotopes27,28,29,30 with high neutron capture cross section, for example, 6Li or 10B. Single crystals and glasses rich in these isotopes are therefore sought as active detector materials31,32,33. However, single crystal detector materials are currently available only in limited sizes, in centimeter scale for commercial Cs2LiYCl6:Ce. Current detector glasses have low light yield (e.g., ~6000 photons/neutron for commercial GS20 lithium glass). Composites bridging these two classes of materials might overcome these issues; they might simultaneously enable larger samples and enhanced light yield34. For example, crystal powder composites of ZnS:Ag and 6LiF have been applied successfully in several neutron scattering facilities, including the Japan Proton Accelerator Research Complex (J-PARC, Japan)35, the Spallation Neutron Source (SNS, United States)36, the ISIS Neutron and Muon Source (ISIS, United Kingdom)37 and the China Spallation Neutron Source (CSNS, China)38. However, it is a totally opaque material that needs to be shaped into a thin film with a certain thickness for balancing photon generation and extraction. Alternatively, HC-TGC materials might overcome all of the above issues: they could offer glass-forming ability, high yield, and optical transparency for facile photon extraction.
Here, we present such HC-TGC materials using a versatile synthesis procedure enabling a broad variety of chemical formulations and microstructural designs. Our approach is based on the solidification of a mixture of melts with a stark contrast in crystallization habit. In this solidification process, one of the melts undergoes a glass transition, whereas the other crystallizes. As a result, the HC-TGC formed having the target crystalline phase. This previously unexplored approach circumvents the limitations of conventional ceramic technology; it avoids extensive protocols of thermal processing and, by this, interference from secondary reactions such as competing phase transitions, crystal growth, or interfacial degradation. In this way, hybrid HC-TGC materials such as oxychloride, oxybromide, and oxyiodide composite systems are obtained, which have thus far been outside of the synthetic capabilities of classical ceramic processing. These HC-TGC materials exhibit superior optical properties and neutron response-ability; as a proof of concept, efficient neutron monitoring and single neutron detection are demonstrated.
The current synthesis procedure benefits from the notable differences in the solidification process of various melts. In particular, melts may solidify by a first order phase transition, forming one or more crystalline phases, or through a glass transition. The latter requires sufficiently deep supercooling, which, in turn, is affected by liquid fragility39 and the difference between the liquidus temperature Tl and the glass transition temperature Tg. The solidification behavior is typically illustrated by using time-temperature-transformation (TTT) diagrams, as illustrated in Fig. 1a. The transition lines drawn within such diagrams represent the practical stability limits of observed phases or material regimes as a function of time and temperature. The melt-crystal transition features a sudden volume change, which often causes micro-cracking, delamination, or residual stress in conventionally processed ceramic products (Fig. 1b). When crystallizing a melt during cooling, crystal nucleation and growth occur simultaneously. As a result, the obtained polycrystalline solid is usually opaque and exhibits larger crystallites and a wider distribution of crystallite sizes. In contrast, the glass transition occurs without a volume jump, and the obtained material is a homogeneous relic of the original liquid. Starting from these distinct differences. We now consider the possibility. When these liquids are combined in such a way that one solidifies into a glass whereas the other crystallizes in similar temperature ranges (but the glass-forming liquids solidify at a somewhat lower temperature as compared to the crystallizing one, see Fig. 1a), synchronous solidification occurs. In this case, the glass (evolving from a viscous, super-cooled liquid) facilitates stress dissipation and contributes to a mechanically robust composite, and crystal growth is limited to the relic structure of the precursor emulsion (Fig. 1c).
Results and discussion
Microstructure of the SrCl2 HC-TGC material
To validate this hypothesis, we designed an oxychloride system composed of crystalline SrCl2 and a borate glass phase, given the different crystallization nature of their precursor melts: SrCl2 has a more ionic character, whereas the borate glass phase is more covalent, network forming. Both melts exhibit similar melting ranges. SrCl2 has a melting temperature of 1147 K, and borate glasses (SrO-B2O3) can be melted at around 1400 K. In a typical synthesis procedure, 100 g of raw materials with the molar ratio of SrCl2:borate glass of 9:1 were homogeneously mixed and co-melted at 1423 K. Interestingly, co-melting with a short duration of ~15 min allowed to obtain a clear melt (the photograph of clear melt is shown in Supplementary Fig. 1); direct solidification of this melt by rapid quenching led to transparent samples (Fig. 2a). In contrast, pure SrCl2 otherwise fabricated by the same process is totally opaque (Supplementary Fig. 2). The sample size can easily reach the scale of 101 cm; in our laboratory, it was limited only by the available mold size. The microstructure of this sample was investigated by confocal optical microscopy, X-ray diffraction (XRD), and Raman spectroscopy. Optical microscopy revealed a dense microstructure with particles with an average, rather homogeneous size of ~10 µm. These particles are separated by a thin boundary layer (Fig. 2b). The thickness of this layer was estimated by selective etching to ~1 µm (Supplementary Fig. 3a, b). XRD confirmed the high crystallinity of the sample, with cubic SrCl2 (PDF 01-075-1623) as the sole crystal phase (Fig. 2c). The corresponding Raman spectrum taken on a single particle exhibited a sharp band located at 184 cm−1, characteristic for cubic SrCl2 (Fig. 2d)40. In contrast, spectra taken in the boundary layer exhibited bands at ~818, 907, and 1236 cm−1, in accordance with the Raman scattering fingerprint of borate glass (inset of Fig. 2d)41. The elemental composition of the particle and of the boundary region was determined by energy dispersive spectroscopy (EDS) (Supplementary Figs. 4 and 5). The particle region was found to be stoichiometric SrCl2, with traces of oxygen, but no other impurities. For the boundary region, Sr, Cl, and O were detected. Raman line scans were collected across three adjacent particles, monitoring the peak height intensity of Raman scattering at 184 cm−1 (The fingerprint peak of SrCl2) and 907 cm−1 (The fingerprint peak of borate glass), as shown in Fig. 2e and Supplementary Fig. 6. These line scans clearly reflect the alternating presence of SrCl2 and of a borate glass phase, with a period of ~10 µm (consistent with the observed average size of the SrCl2 particles). A real Raman mapping analysis further confirmed that SrCl2 particles are homogeneously distributed and isolated by a thin borate glass layer (Fig. 2f). Finally, confocal microscopy provided a similar view in three dimensions (Fig. 2g). Taken together, these data clearly show that the current HC-TGC is composed of cubic SrCl2 crystals surrounded by thin layers of a borate glass phase. The overall crystallinity reaches up to about 85 vol%.
a Sample Photograph, (b) optical micrograph, (c) XRD pattern of the SrCl2 HC-TGC material. d Raman spectra taken on particle and boundary regions, respectively. The inset shows the Raman spectrum of the SrCl2 HC-TGC sample in the range of 700 ~ 1400 cm−1. e Raman line scans crossing three particles, monitoring the Raman scattering intensity near 184 cm−1 and 907 cm−1, respectively. f Areal Raman mapping, and (g) confocal microscopic imaging of the SrCl2 HC-TGC sample.
Formation mechanisms of the SrCl2 HC-TGC material
We investigated the formation mechanism of HC-TGC materials via the co-solidification of the mixture of melts in two regards, i.e., understanding how the highly regular SrCl2-borate glass microstructure evolves, and which factors govern the resultant composite’s optical transparency. First, the formation mechanism of SrCl2 HC-TGC was studied. To this end, we performed molecular dynamic simulations on the structure evolution from melt to solid. Figure 3a presents the atomic configurations and packing manner of the SrCl2-borate glass system at various temperatures from 1500 K to 300 K. Chemical heterogeneity can be observed at various stages from melt to solid. Snapshots of structural models indicate that the heterogeneity evolves from [-Sr-Cl-] and [-B-O-] clusters. These clusters are supposed to behave as the embryos of the crystalline SrCl2 and borate glass phases, respectively. In-situ high-temperature confocal microscopy was employed to study the solidification process of HC-TGC materials (Fig. 3b). Upon cooling from 1073 K – 1042 K, SrCl2 particles precipitated, and their size rapidly increased. In contrast, no obvious particles can be observed in the pure borate glass system (Fig. 3c). Based above studies, the possible formation mechanism of SrCl2 HC-TGC can be summarized as follows. Firstly, the melts with a stark contrast in crystallization habits need to be mixed sufficiently to ensure that crystal precipitation occurs uniformly. Secondly, during solidification, the target SrCl2 crystal rapidly precipitates from the mixture of melts. In contrast, the melt contains [-B-O-] clusters is repelled from the crystal and remains in liquid throughout the crystal precipitation process because of its low tendency to crystallization. As a result, SrCl2 crystal particles can be well-protected by the soft glass phase. Thirdly, when the temperature is further decreased, the glass phase gradually loses its mobility and solidifies at the boundary.
a Atomic structure of SrCl2-borate glass system at various temperatures from 1500 K to 300 K. b-c In-situ high-temperature confocal laser scanning microscope photographs of the SrCl2 HC-TGC and the pure borate glass at various temperatures during the solidification process. d The nominal compositions of the SrCl2 HC-TGC and composites with different mole ratios of crystalline SrCl2 and borate glass. e Typical microstructures of samples A, B, C, D, E, and F are marked in (d).
To test the tailor-ability of the HC-TGC microstructure, a series of samples was produced by varying the mole ratio of SrCl2:borate glass, as denoted “A” to “F” in Fig. 3d. The corresponding nominal compositions of the samples are listed in Supplementary Table 1. Sample microstructures were studied after etching the crystalline fraction of SrCl2 (Fig. 3e). The volume ratio of crystalline SrCl2 and borate glass can be finely tailored from ~85% (A) to 80% (B), 60% (C), and 45% (D) (as shown in Supplementary Fig. 7). The average crystal size of SrCl2 changed from 16 to 13, 10 and 6 μm, respectively. In samples E and F, no obvious SrCl2 crystal was observed. Following the demonstration of tunable microstructure, the origin of optical transparency was investigated. SrCl2 crystal (cubic) and the borate glass phase are both isotropic. The refractive indices nD were measured to be 1.65042 and 1.643 at 589 nm (sodium D-line), respectively. The microstructures with small birefringence and refractive index difference are expected to simultaneously contribute to the excellent optical transmission of the SrCl2 HC-TGC material (as shown in Supplementary Fig. 8). For the purpose of demonstration, on these lines, we produced a range of dense HC-TGC materials involving chlorides (LiCl, NaCl, KCl, RbCl, CsCl, CaCl2, BaCl2, KSr2Cl5, CsSrCl3, and CsCaCl3), bromides (SrBr2), and iodides (CsI) (Supplementary Figs. 9–20).
Optical and scintillation properties of the Eu-doped SrCl2 HC-TGC material
The interest in HC-TGC materials involving unusual material combinations such as those considered here arises from the optical properties of the crystal phase, combined with the processing capabilities of direct melt-solidification and the protective function of the interfacial glass layer. In particular, chlorides, bromides, and iodides exhibit a low phonon energy (e.g., ~200 cm−1 for crystalline SrCl2)43 and wide optical transmission windows extending into the mid-infrared spectral range. This facilitates internal quantum emission as well as subsequent light extraction. Rare earth species are typically used as internal emission centers. In the present case, Europium (Eu) was employed as the active dopant. Figure 4a shows the photoluminescence spectra of the Eu-doped composite with variable crystal volume fraction. The intense blue emission band at ~402 nm and the weak red emission bands at ~590, ~610, and ~651 nm are ascribed to the characteristic 4f65d1 → 4f7(8S7/2) electronic transition of Eu2+ and 5D0 → 7F0,1,2,3 transitions of Eu3+44,45. The Eu-doped SrCl2 HC-TGC material (sample A) exhibits the strongest blue emission (~150 times higher in intensity than the red emission). We assume that this is a result of the selective incorporation of Eu2+ into crystalline SrCl2. The weak red emission, on the other hand, comes from the residual Eu3+ in the boundary glass phase. The corresponding excitation-emission map reveals no significant emission shift when the excitation wavelength is varied, indicating that Eu2+ centers occupy a single structural site (Fig. 4b). In addition, same as the Eu-doped SrCl2 crystal, the emission of Eu-doped SrCl2 HC-TGC material also exhibits narrowband feature. The emission decay feature was also confirmed by dynamic luminescence spectroscopy, which shows a single exponential function (Fig. 4c). The average decay lifetime was calculated at ~400 ns, which is typical for the 5d-4f transition of Eu2+. The bright emission and fast decay dynamics imply great potential as a scintillating material. Figure 4d, e exhibit the scintillating emission of Eu-doped composites with variable volume content of SrCl2 crystal, under excitation with X-ray and α-particle irradiation, respectively. Among the sample series, the Eu-doped SrCl2 HC-TGC material (sample A) exhibits the brightest scintillating luminescence, with an observed intensity of the emission peak exceeding that of commercial BGO crystals by a factor of 8 (X-ray excitation) and 25 (α-particles excitation). Figure 4f summarizes the emission intensity observed for various composites as a function of crystalline SrCl2 volume fraction. The X-ray induced blue emission sharply increases with higher crystallinity. Together with the high optical transparency of Eu-doped SrCl2 HC-TGC material (Supplementary Fig. 8), the experimental results firmly demonstrate that the proposed approach for HC-TGC materials provides an effective strategy towards transparent optical composites with performance close to that of a corresponding single crystal.
a Photoluminescence of Eu-doped composites with different volume ratios of crystalline SrCl2 and borate glass. The inset is the photograph of the Eu-doped SrCl2 HC-TGC material under the excitation of 365 nm. b Excitation-emission map of Eu-doped SrCl2 HC-TGC material. c Decay curve of Eu-doped SrCl2 HC-TGC at 403 nm emission under excitation of 330 nm ultraviolet light. d X-ray and (e) α-particle induced luminescence of Eu-doped composites with different volume ratios of crystalline SrCl2 and borate glass. The commercial BGO product is also shown for comparison. f SrCl2 crystal volume ratio-dependent emission intensity for various composites under X-ray excitation.
HC-TGC derived detector for thermal neutron detection
More excitingly, we demonstrate that the present composite material enables neutron detection, a significant challenge for current scintillator materials. The principle relies on the cooperative action of the interfacial glass phase and the active SrCl2 crystals. It involves detecting the scintillating emission generated by neutron interaction. As illustrated in the inset of Fig. 5a, neutrons are blocked by 10B isotope species, which are part of the intergranular glass phase via 10B(n, α)7Li nuclear reaction. Assumedly, these releases charged 7Li and α-particle46. The resultant particles ionize Eu2+ in crystalline SrCl2 directly or indirectly through secondary electrons, generating visible scintillating emission that can be detected by optical spectroscopy. To evaluate this hypothesis, we systematically studied neutron detection properties in various samples. Excitingly, a strong neutron signal can be detected in HC-TGC, with a light yield of about 4700 photons/neutron (Fig. 5a). It is necessary to point out that no neutron signal can be detected neither in pure borate glass nor in SrCl2 crystal (as shown in Supplementary Figs. 21 and 22), demonstrating that only the interaction of the two components in the composites enables its functionality.
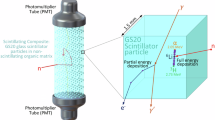
a The energy spectrum of the thermal neutron obtained by using the SrCl2 HC-TGC material. The inset is the illustration of the 10B(n, α)7Li reaction with a thermal neutron in the SrCl2 HC-TGC material. b Energy deposition in SrCl2 HC-TGC material with different volume percentages of crystalline SrCl2. c, d Comparison of the energy deposition distribution in the composites with different configurations. Purple represents the crystal phase, and orange represents the glass phase. e Schematic diagram of the neutron detector derived from the HC-TGC and its application for neutron detection in CSNS. f ToF spectrum obtained from the detector in (e). g A snapshot of the signal in the time window of ~6 ms. The inset is a typical single neutron event.
To shed more light on the physical mechanism behind the robust neutron detection ability, we made a detailed analysis of the neutron-composite interaction using the Monte Carlo approach with the Geant4 toolkit47,48,49. As illustrated in Supplementary Fig. 23, the nuclear reaction products (α-particles and 7Li nucleus) mediated by 10B(n, α)7Li reaction are highly active with kinetic energies of ~1.47 MeV and ~0.83 MeV, respectively. Thus, their relaxation processes and the local environment may govern the energy deposition efficiency. The relaxation processes of α-particles and 7Li nucleus were analyzed, and their mean free path lengths were calculated to be ~5 µm and ~2 µm, respectively. Based on this, the composite configuration-dependent neutron deposition can be obtained and summarized in Fig. 5b. It can be observed that the energy deposition efficiency of neutrons strongly relies on the glass/crystal ratio in the composite (and, by this, on the crystallite thickness). It reaches the peak level of 1.09 MeV/neutron with the crystalline SrCl2 ratio of 92 vol% (with a thick SrCl2 blocking layer). Figure 5c, d present the distribution of the energy deposition in the composite with two different configurations. In the HC-TGC, the energy collection efficiency is rather high, and almost 61% of the energy released via nuclear reaction can be successfully harvested (Fig. 5c). In stark contrast, in the composite with a relatively low crystal volume fraction (and in turn, with a thick borate glass blocking layer, Fig. 5d), most of the α-particles and 7Li nucleus are dissipated in the borate glass, and only ~18% of energy can be captured for generating the photon signal. In the case of pure crystalline SrCl2, because of the absence of the borate glass, the neutron capture ability is significantly reduced compared with that of HC-TGC material, resulting in a dramatic decrease in detection efficiency. Together, these observations confirm that the unique, spatially homogeneous microstructure of the HC-TGC and the collective action of the glass and of the crystal phase enable synergistic neutron transformation and energy deposition, resulting in a greatly improved neutron detection efficiency.
From these material observations, a neutron detector device was elaborated (as illustrated in Fig. 5e). The neutron detector was built with SrCl2 HC-TGC material, a photomultiplier tube (PMT), a high-voltage power supply, a multichannel analyzer (MCA), and a computer. In this detector, the SrCl2 HC-TGC material was processed into a round shape with the same diameter as the PMT and covered with an aluminum foil reflector to increase the collection efficiency of scintillation photons. The covered HC-TGC has one window to couple with the PMT using optical silicone grease. The PMT was driven using a high-voltage power supply, and the signals from the PMT were collected by an MCA. Finally, the results from the MCA, such as pulse shape and pulse-height spectrum, were displayed on the computer. This detector was applied for online monitoring of neutrons in CSNS (Fig. 5e). The detector is located at ~11 m away from the radiation source. Figure 5f shows a typical time-of-flight (ToF) spectrum obtained from the detector. The intense peak at ~0 ms originates from γ rays, which arrive at the detector first. A characteristic broad band centered at ~5 ms is associated with the thermal neutron group. Because the average flight speed of thermal neutrons is ~2200 m·s−1, the theoretical time interval between γ rays and the thermal neutron group should be ~5 ms, which is well consistent with the experiment results. The shoulder band close to the gamma signal peak originates from fast neutrons. The above results firmly demonstrate that utilizing an HC-TGC derived detector enables the achievement of rich radiation information and clearly distinguishes neutrons from other radiation. In addition, the detector permits the capture of a single neutron signal. Figure 5g shows a snapshot of the arrived signal in the time window of ~6 ms, and attractively, around 300 ~ 400 single neutron events can be screened within 1 ms. The enlarged Figure in Fig. 5g presents a typical single neutron signal. Furthermore, it should be noted that all of the chemical components in the device are in natural abundance. This contrasts with a conventional neutron detector, which strictly relies on enriched isotopes.
In summary, we have presented and demonstrated a strategy to construct HC-TGC materials from the mixture of melts with a stark contrast in crystallization habit. Significantly, the strategy is general and can be applied to various unusual HC-TGC materials such as oxychloride, oxybromide, and oxyiodide composite systems. By using these HC-TGC materials, an efficient neutron detector has been developed, and its practical applications for neutron monitoring have been demonstrated. Our findings are expected to generate a new composite with promising applications in photonics, nuclear, and other related fields.
Methods
Material synthesis
HC-TGC materials were fabricated through the melt-quenching method. The raw materials were mixtures of halide crystal powders (e.g., SrCl2 powder) and halide oxide glass powders (e.g., borate glass with the nominal composition of SrO:B2O3 = 40:60 mol%) with designed molar percentages. These mixtures were put into the alumina crucibles and melted at 1423 K for 1 ~ 15 min. Small HC-TGC samples (e.g., 5 g) can be fabricated within a short duration (1 ~ 5 min). Large HC-TGC samples (e.g., 100 g) should be fabricated for a long duration (about 15 min). Then, the melt was cast into the cold stainless-steel molds and cooled to room temperature naturally. Finally, the HC-TGC was fabricated and processed into various shapes by a wire-cutting machine. In addition, the raw materials and melting temperatures of other HC-TGC materials involving chlorides (LiCl, NaCl, KCl, RbCl, CsCl, CaCl2, BaCl2, KSr2Cl5, CsSrCl3, and CsCaCl3), bromides (SrBr2), and iodides (CsI) are shown in Table 1.
Material characterization
The X-ray diffraction was tested on the PANalytical X’Pert PRO X-ray instrument, and block HC-TGC samples with polished surfaces were used for measurement. Raman spectra were tested on the Renishaw InVia laser confocal Raman spectrometer. The microstructures of the HC-TGC materials were studied on an SEM (ZEISS, EVO, Germany) equipped with an energy-dispersive X-ray spectrometer (Oxford Instruments, X-Max, U.K.) and Electron probe X-ray micro-analyzer. The HC-TGC samples were etched by using a mixture liquid of ethanol (90 vol%) and water (10 vol%). The confocal microscope was imaged on a laser confocal microscope (Lecia, DM5500 Q, Germany, and Lecia, TCS SP8, Germany). Pr3+ dopant with red emission was used as the fluorescence probe, and 405 nm laser was employed as the excitation source. The excitation and photoluminescence spectra were recorded using a fiber spectrometer (Ocean Optics, Maya 2000 Pro, USA) equipped with a xenon arc lamp. The HC-TGC samples were fine-polished on both sides, with a thickness of ~1 mm. The transmission mode was used for this measurement. The decay curve was recorded using a fluorescence spectrophotometer (Edinburgh Instrument Ltd., FLS920, U.K.). The monitoring wavelength is 403 nm, and the excitation wavelength is 330 nm. The radioluminescence spectra and α-particle excited luminescence spectra were measured in a special facility, which consisted of a miniature X-ray tube (AMPTEK Inc., Mini-X-OEM, USA) with Rh target X-ray tube and 241Am as α-particle sources, and a fiber spectrometer (Ocean Optics, Maya 2000 Pro, USA) as a detector. The tube voltage is 40 keV, and the range of tube current was 0 ~ 100 μA. The HC-TGC samples were fine-polished on both sides, with a thickness of ~1 mm. The transmission mode was used for this measurement. The neutron-related properties were measured at the China Spallation Neutron Source (CSNS). The system consists of a photomultiplier tube assembly (HAMAMATSU, H1949-51, Japan), a high-voltage power supplier (ISEG, SHR High Precision High Voltage Power Supply, Germany), a multichannel analyzer (TOFTEK, DAQBOX-32, China), and a computer. The operating voltage of the photomultiplier tube assembly was −1700 V. The threshold of the multichannel analyzer was set at 7.5 mV for energy spectrum measurement. The integral time of the multichannel analyzer needed to be adjusted according to the decay time of the dopants.
Molecular dynamics simulation
The LAMMPS package was employed to simulate the glass structure. The system was composed of 3017 atoms. The initial configurations were generated with experimental density using packmol package. Interactions between atoms were simulated using Born-Mayer potential, and the charges of B, Sr, Cl, and O were set to +1.8, +2.0, −1.0, and −1.412, respectively. The timestep was set to 1 fs, and the Columb interactions were solved using the particle-particle-particle-mesh method. All systems were heated for 200 ps at 4000 K using the NVT ensemble after energy minimization to eliminate abnormal stacking between atoms. Subsequently, the system was cooled at a rate of 1 K ps−1 under the NPT ensemble. During the quenching process, a 100 ps NVE ensemble was performed every 300 K to gather enough configurations and snapshots. Meanwhile, the phase separation was investigated, assuming that the thermal expansion coefficient was fixed to be the value of 250 × 10−7 K−1. In the final period, a 100 ps NVE at 300 K was used to obtain enough trajectories for structural analysis.
Monte Carlo simulation
The Monte Carlo method was used to study the interaction between neutron and HC-TGC materials. The energy deposited distribution on HC-TGC was investigated with the help of the Geant4 toolkit, and the resulting data were analyzed using ROOT applications. C + + language was used to set the X-ray source and the model of HC-TGC materials. The energy of the thermal neutron is set at 0.025 eV, which is in thermal equilibrium with the surrounding temperature (290 K). The HC-TGC model was set to be 1 mm thick and 1 × 1 cm2 large. To reduce the computational time of simulation, the structures of the HC-TGC model were simplified as periodic layer structures of the halide crystal phase and glass phase (as shown in Supplementary Fig. 23). The unit thickness of the halide crystal phase and glass phase is 10 μm. The layer thickness of the halide crystal phase in the unit can be switched from 0.1 μm (1 vol% of halide crystal phase in composite) to 9.9 μm (99 vol% of halide crystal phase in composite). The energy deposited distribution was investigated by using the default physics lists, which can be selected in Geant4 by referring to the Geant4 Physics Reference Manual. The event number in each run was set to be 105.
References
Sun, K. et al. Three-dimensional direct lithography of stable perovskite nanocrystals in glass. Science 375, 307–310 (2022).
Huang, X. et al. Reversible 3D laser printing of perovskite quantum dots inside a transparent medium. Nat. Photonics 14, 82–88 (2019).
Tessler, N., Medvedev, V., Kazes, M., Kan, S. & Banin, U. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 295, 1506–1508 (2002).
Steckel, J. S., Coe-Sullivan, S., Bulović, V. & Bawendi, M. G. 1.3 μm–1.55 μm tunable electroluminescence from PbSe quantum dots embedded within an organic device. Adv. Mater. 15, 1862–1866 (2003).
Llordés, A., Garcia, G., Gazquez, J. & Milliron, D. J. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites. Nature 500, 323–326 (2013).
Llordés, A. et al. Linear topology in amorphous metal oxide electrochromic networks obtained via low-temperature solution processing. Nat. Mater. 15, 1267–1273 (2016).
Zhang, D. et al. Highly efficient phosphor-glass composites by pressureless sintering. Nat. Commun. 11, 2805 (2020).
Lin, H., Hu, T., Cheng, Y., Chen, M. & Wang, Y. Glass ceramic phosphors: towards long-lifetime high-power white light-emitting-diode applications-a review. Laser Photonics Rev. 12, 1700344 (2018).
Zhang, R. et al. A new-generation color converter for high-power white LED: transparent Ce3+:YAG phosphor-in-glass. Laser Photonics Rev. 8, 158–164 (2014).
Tao, G., Stolyarov, A. M. & Abouraddy, A. F. Multimaterial fibers. Int. J. Appl. Glass Sci. 3, 349–368 (2012).
Yan, W. et al. Thermally drawn advanced functional fibers: new frontier of flexible electronics. Mater. Today 35, 168–194 (2020).
Angel, J. R. P. Very large ground-based telescopes for optical and IR astronomy. Nature 295, 651–657 (1982).
Wanner, G. Space-based gravitational wave detection and how LISA pathfinder successfully paved the way. Nat. Phys. 15, 200–202 (2019).
Liu, X., Zhou, J., Zhou, S., Yue, Y. & Qiu, J. Transparent glass-ceramics functionalized by dispersed crystals. Prog. Mater. Sci. 97, 38–96 (2018).
Allix, M. et al. Highly transparent BaAl4O7 polycrystalline ceramic obtained by full crystallization from glass. Adv. Mater. 24, 5570–5575 (2012).
Ma, X. et al. Pressureless glass crystallization of transparent yttrium aluminum garnet-based nanoceramics. Nat. Commun. 9, 1175 (2018).
Kruk, A. Optical and structural properties of arc melted Ce or Pr-doped Y2O3 transparent ceramics. Ceram. Int. 43, 16909–16914 (2017).
Milisavljevic, I. et al. Crystallization of glass materials into transparent optical ceramics. Int. Mater. Rev. 68, 648–676 (2022).
Karpukhina, N., Hill, R. G. & Law, R. V. Crystallisation in oxide glasses-a tutorial review. Chem. Soc. Rev. 43, 2174–2186 (2014).
Yasui, N., Ohashi, Y., Kobayashi, T. & Den, T. Development of phase-separated scintillators with light-guiding properties. Adv. Mater. 24, 5464–5469 (2012).
Boyer, M. et al. Enhanced transparency through second phase crystallization in BaAl4O7 scintillating ceramics. Cryst. Growth Des. 16, 386–395 (2015).
Dymova, M. A., Taskaev, S. Y., Richter, V. A. & Kuligina, E. V. Boron neutron capture therapy: current status and future perspectives. Cancer Commun. 40, 406–421 (2020).
Engel, E. M. & Danagoulian, A. A physically cryptographic warhead verification system using neutron induced nuclear resonances. Nat. Commun. 10, 4433 (2019).
Van der Ende, B. M., Li, L., Godin, D. & Sur, B. Stand-off nuclear reactor monitoring with neutron detectors for safeguards and non-proliferation applications. Nat. Commun. 10, 1959 (2019).
Hosseini, M., Arif, M., Keshavarz, A. & Iglauer, S. Neutron scattering: a subsurface application review. Earth Sci. Rev. 221, 103755 (2021).
Kardjilov, N., Manke, I., Woracek, R., Hilger, A. & Banhart, J. Advances in neutron imaging. Mater. Today 21, 652–672 (2018).
Pietropaolo, A. et al. Neutron detection techniques from μeV to GeV. Phys. Rep. 875, 1–65 (2020).
Chica, D. G. et al. Direct thermal neutron detection by the 2D semiconductor 6LiInP2Se6. Nature 577, 346–349 (2020).
Runkle, R. C., Bernstein, A. & Vanier, P. E. Securing special nuclear material: recent advances in neutron detection and their role in nonproliferation. J. Appl. Phys. 108, 111101 (2010).
Li, P. et al. Large scale BN-perovskite nanocomposite aerogel scintillator for thermal neutron detection. Adv. Mater. 35, 2209452 (2023).
Cieślak, M., Gamage, K. & Glover, R. Critical review of scintillating crystals for neutron detection. Crystals 9, 480 (2019).
Van Eijk, C. W. E. Inorganic scintillators for thermal neutron detection. Radiat. Meas. 38, 337–342 (2004).
Van Eijk, C. W. E. Inorganic scintillators for thermal neutron detection. IEEE Trans. Nucl. Sci. 59, 2242–2247 (2012).
Chen, L. et al. Nanoscale Gd2O2S:Tb scintillators for high-resolution fluorescent imaging of cold neutrons. ACS Appl. Nano Mater. 5, 8440–8447 (2022).
Nakamura, T. et al. 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC) (IEEE, 2014).
Wang, C. L. et al. 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC) (IEEE, 2012).
Mauri, G., Sykora, G. J., Schooneveld, E. M. & Rhodes, N. J. Enhanced position resolution for ZnS:Ag/6LiF wavelength shifting fiber thermal neutron detectors. Eur. Phys. J. 136, 286 (2021).
Huang, C. et al. Performance test of different silicon photomultipliers for the 6LiF:ZnS(Ag) based neutron detectors of CSNS. J. Instrum. 17, T12008 (2022).
Qiao, A. et al. A metal-organic framework with ultrahigh glass-forming ability. Sci. Adv. 4, eaao6827 (2018).
Denham, P. A., Morse, P. L. R. & Wilkinson, G. R. The phonon dispersion curves, infra-red and Raman spectra of SrCl2. J. Phys. C 6, 2066–2075 (1973).
Winterstein-Beckmann, A., Möncke, D., Palles, D., Kamitsos, E. I. & Wondraczek, L. Structure-property correlations in highly modified Sr, Mn-borate glasses. J. Non Cryst. Solids 376, 165–174 (2013).
Li, H. H. Refractive index of alkaline earth halides and its wavelength and temperature derivatives. J. Phys. Chem. Ref. Data 9, 161–290 (1980).
Sadoc, A., Moussa, F. & Pepy, G. The lattice dynamics of SrCl2. J. Phys. Chem. Solids 37, 197–199 (1976).
Luo, Q., Qiao, X., Fan, X. & Zhang, X. Luminescence properties of Eu2+-doped glass ceramics containing SrF2 nanocrystals. J. Am. Ceram. Soc. 93, 2684–2688 (2010).
Qiao, J. et al. Near-infrared light-emitting diodes utilizing a europium-activated calcium oxide phosphor with external quantum efficiency of up to 54.7%. Adv. Mater. 34, 2201887 (2022).
Chuirazzi, W., Craft, A., Schillinger, B., Cool, S. & Tengattini, A. Boron-based neutron scintillator screens for neutron imaging. J. Imaging 6, 124 (2020).
Agostinelli, S. et al. Geant4-a simulation toolkit. Nucl. Instrum. Methods Phys. Res. A 506, 250–303 (2003).
Allison, J. et al. Geant4 developments and applications. IEEE Trans. Nucl. Sci. 53, 270–278 (2006).
Allison, J. et al. Recent developments in Geant4. Nucl. Instrum. Methods Phys. Res. A 835, 186–225 (2016).
Acknowledgements
S.L. and D.W. contributed equally to this work. The authors gratefully acknowledge financial support from the National Science Fund for Distinguished Young Scholars (62125502), the National Natural Science Foundation of China (52302002 and 62305115), the Foundation of State Key Laboratory of Reactor System Design Technology, the Large Scientific Facility Open Subject of Songshan Lake, Dongguan, Guangdong, the Research Project supported by State Key Lab of Luminescent Materials and Devices, South China University of Technology (Skllmd-2023-07 and Skllmd-2024-20).
Author information
Authors and Affiliations
Contributions
S.Z. and S.L. designed the research. S.L., D.W., and J.T. performed the research on material fabrication. S.L., Z.L., and H.I. performed the research on theoretical simulations. B.T. and Z.S. performed the research on neutron detection. S.L., D.W., and B.T. analyzed the data. S.L., D.W., Z.L., L.W., J.Q., and S.Z. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Haohai Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lv, S., Wang, D., Tang, J. et al. Transparent composites for efficient neutron detection. Nat Commun 15, 6746 (2024). https://doi.org/10.1038/s41467-024-51119-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51119-w