Abstract
Understanding the Li-ions conduction network and transport dynamics in polymer electrolyte is crucial for developing reliable all-solid-state batteries. In this work, advanced nano- X-ray computed tomography combined with Raman spectroscopy and solid state nuclear magnetic resonance are used to multi-scale qualitatively and quantitatively reveal ion conduction network of poly(ethylene) oxide (PEO)-based electrolyte (from atomic, nano to macroscopic level). With the clear mapping of the microstructural heterogeneities of the polymer segments, aluminium-oxo molecular clusters (AlOC) are used to reconstruct a high-efficient conducting network with high available Li-ions (76.7%) and continuous amorphous domains via the strong supramolecular interactions. Such superionic PEO conductor (PEO-LiTFSI-AlOC) exhibites a molten-like Li-ion conduction behaviour among the whole temperature range and delivers an ionic conductivity of 1.87 × 10−4 S cm−1 at 35 °Ϲ. This further endows Li electrochemical plating/stripping stability under 50 μA cm−2 and 50 μAh cm−2 over 2000 h. The as-built Li|PEO-LiTFSI-AlOC|LiFePO4 full batteries show a high rate performance and a capacity retention more than 90% over 200 cycling at 250 μA cm−2, even enabling a high-loading LiFePO4 cathode of 16.8 mg cm−2 with a specific capacity of 150 mAh g−1 at 50 °Ϲ.
Similar content being viewed by others
Introduction
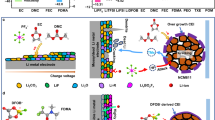
Revealing the Li-ion conduction network and transport dynamics of Li-ions in the bulk PEO polymer electrolyte is still a great challenge1,2,3,4,5,6. This is highly important to reconstruct and further enhance the polymer electrolyte to build advanced all-solid-state Li batteries. The Li-ion transportation typically occurs by the intrachain or interchain hooping inside the PEO-based electrolytes, with the processes of breaking/forming lithium–oxygen (Li–O) bonds7,8. However, the ethylene oxide (EO) units with a high donor number will have a strong complexing ability to impede the gradual replacement of the ligands for the solvation of Li-ions9. Some literature has reported the participation of anions in Li-ion coordination, which may further affect the transport properties of material10,11. In addition, the conductivity and mobility of Li+ are extremely dependent on the segmental mobility of PEO12. Due to intrinsic semi-crystallinity, the overall segment motion dynamics is sluggish at room temperature13. Although there are various processing methods to improve the ionic conductivity, a spontaneous crystallization process with flexible chains transforming into rigid conformational ordered segments still exists during the operation of the battery. The accompanying decrease in ionic conductivity will lead to a rapid decline in battery performance14. Furthermore, increasing the operating temperature for typical PEO-Li salt complexes can also reach acceptable ionic conductivity (≥ 10−4 S cm−1). However, the operating temperature usually should be higher than the melting point of ~ 60 °Ϲ. At such elevated temperatures, PEO solid electrolyte would be in the molten state and lose its mechanical behavior. This will, in turn, induce severe safety risks due to the easier formation of metallic Li dendrites.
PEO exhibits a very low ionic conductivity of 10−6 S cm−1 at room temperature with a molar ratio of Li:EO = 0.05 ~ 0.12, which is related to polymer chains, Li-ions, and anions, as well as the existence of multiple scales ion conduction15,16. This raises the importance of a comprehensive understanding of Li-ion conduction among the whole polymer network from the local atomic nano level to the macroscopic level. Previous efforts are usually focused on the local atomic and nano-level among the different components/phases of polymer electrolytes17,18. For instance, nuclear magnetic resonance (NMR) techniques could distinguish and trace the Li-ion transportation dynamics among different components/phases by solid-state Li NMR and 6Li → 7Li isotope replacement19. However, the ionic conductivity of solid electrolytes is usually measured by macroscopic samples. How Li-ions transport inside the PEO at the macroscopic level and the relationship between macroscopic ion transport and microscopic ion transport are unclear. It requires the development of a method to obtain macroscopic information and integrate it with microscopic information. X-ray computed tomography (XCT) is a non-destructive technology to obtain the three-dimensional morphology of materials at the sub-micron scale in battery studies20,21. For example, McDowell et al. used operando XCT to observe the loss and reconfiguration of interfacial contact between lithium and the solid-state electrolyte (Li10SnP2S12)22. Bruce et al. also used in situ XCT and spatially mapped X-ray diffraction to study the mechanism for Li dendrite growth through ceramic electrolytes23. However, the three-dimensional imaging of materials via XCT typically relied on the relationship between X-ray absorption and sample density. Due to the very small density difference between the crystallized state (~ 1.3 g cm−3) and the amorphous state of PEO ( ~ 1.2 g cm−3), it is still a challenge to use conventional XCT to visualize the complete PEO phase structure.
Furthermore, elucidating the intricate relations between macroscopic ionic conductivity and the global Li-ion conduction network is the key to providing reliable strategies for the optimization and development of high-performance solid-state electrolyte24,25. Current efforts to improve polymer electrolytes include nanosized nonconductive fillers and Li+ solid electrolytes into the PEO polymer electrolytes26,27,28,29,30,31. The mechanism for enhancing ionic conductivity has not been fully clarified yet, which results from the unclear interactions of components and complex Li+ conduction mechanisms of these composite electrolytes. Metal-oxo molecular clusters are atomically precise molecules with uniform size adjustable molecular organic shells and interesting supramolecular chemistry, which have attracted widespread attention in functional material synthesis, catalysis, electronic devices, and electrochemical energy storage and conversion32,33,34,35. The atomically precise supramolecular chemistry allows the metal-oxo molecular cluster to be a suitable research model.
In this work, we investigated metal-oxo molecular clusters, aluminum-oxo molecular rings (denoted as AlOC) with atomically precise supramolecular chemistry, to fabricate a superionic PEO conductor. The supramolecular interactions could regulate microstructural heterogeneities of polymer electrolytes to reconstruct the crystallization and amorphous PEO polymer segments to enhance the Li-ion conduction and transportation. We employed high spatial resolution and tunable propagation phase contrast XCT to visualize and quantify microstructural heterogeneities of polymer electrolytes. Combined with Raman mapping technology, a complete visualization of the Li-ion concentration distribution can be realized among the microstructural heterogeneities of the crystallization and amorphous PEO polymer segments. Essentially, conventional PEO-LiTFSI polymer electrolyte has a disconnected amorphous phases ion transport network (the spatial proportion of 33.3%) with low available Li-ions (only 39.4%). This finding can well answer the long and puzzling question quantitatively of why conventional PEO polymer electrolyte exhibits such low Li-ion conductivity at room temperature. For PEO-LiTFSI-AlOC superionic PEO conductor, a highly efficient conducting network in which about 64.6 % of the whole region is occupied by continuous amorphous phases. The available Li-ions in the amorphous Li-ion conducting polymer network are also increased to 76.7 %. Such superionic PEO conductors exhibited a molten-like Li-ion conduction behavior among the whole temperature range and delivered an ionic conductivity approaching 1.87 × 10−4 S cm−1 at 35 °Ϲ. It further endows a stable Li electrochemical plating/stripping of a capacity of 50 μAh cm−2 at 50 μA cm−2 over 2000 h at 35 °Ϲ. The as-built Li|PEO-LiTFSI-AlOC|LiFePO4 full batteries show a high rate performance and long cycling performance with capacity retention more than 90 % and an average coulombic efficiency higher than 99.9 % over 200 cycling at 25 μA cm−2 at 35 °Ϲ, even enabling a high-loading LiFePO4 cathode of 16.8 mg cm−2 with a specific capacity of 150 mAh g−1 at 50 °Ϲ. Our strategy provides valuable insight to characterize ion conduction in solid-state electrolytes and to progress all-solid-state lithium metal batteries.
Results and discussion
Supramolecular interactions between EO unites/anions and AlOC
We introduce a kind of metal-oxo molecular cluster, aluminum-oxo rings (denoted as AlOC), to modify the microstructural heterogeneities of PEO-based polymer electrolyte to fabricate superionic PEO conductor (PEO-LiTFSI-AlOC). In detail, AlOC is constructed with inorganic Al8(OH)8 as cores and two types of organic ligands (R1: 4-methylpyrazole and R2: benzoate) as organic shells (Fig. 1a)36. The carboxylic acid part of benzoic acid and the N atom of 4-methylpyrazole act as a bridge, linking metal ions to form a ring. Therefore, the aromatic nucleus is oriented outwards of the molecular ring. Moreover, six Al8 rings will occupy the six faces of the cube to make a perfect (Al8)6 nanocage under π − π stacking interactions between adjacent rings with both mesoporous cages (21.19 Å) and microporous apertures (7.8 Å). Interaction region indicator (IRI) calculations on the guest-cluster interaction indicate there are substantial host–guest interactions that exist between the ligands and LiTFSI (Fig. 1b). The center of the aluminum molecular ring surrounded by four organic ligands (4-methylpyrazole) (site I) and the microporous channels between the nanocages (site II) could capture TFSI−. This results in a torsion of the organic ligand (Supplementary Discussion 1). In site I, TFSI− is trapped in the middle of the molecular ring by twists in the four 4-methylpyrazole ligands. TFSI− will also be captured in the micropore channel, site II, close to the benzoate and 4-methylpyrazole ligands. In addition, compared with site II, there is a hydrogen bond between the hydroxyl group and TFSI− at site I, which is more conducive to the adsorption of the guest. Therefore, the adsorption energy of site I for TFSI− is − 2.9 eV due to multiple interaction forces, which is lower than that at site II (− 2.0 eV). So both sites can make the N–Li bond lengthened (site I: 2.0 Å, site II: 2.1 Å, init. 1.9 Å). The lengthened N–Li bond reduces the ionic bond strength of LiTFSI and induces the dissociation of lithium salt in the polymer electrolyte. Furthermore, the significant adsorption of anions by AlOC may reduce the strength of the Coulomb interactions between anions and cations.
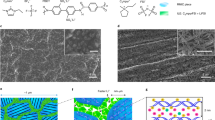
a the nanocage assembly process of Al8(OH)8(R1)8(R2)8 (R1 = 4-methylpyrazole, R2 = benzoate) and the simulated adsorption sites of lithium bis(trifluoromethane sulfonyl) imide (LiTFSI) on the nanocage. b the adsorption simulation details for Site I of the aluminum-oxo ring and Site II of the assembled mesoporous cage. In order to highlight the structural interaction, the non-interaction parts are virtualized by space-filling mode, while the important interaction sites and guests are represented by standard ball-and-stick mode. More details can be seen in Note S1. c the schematic of the supramolecular interaction of aluminum-oxo rings (AlOC) with poly(ethylene) oxide (PEO) segment. d the stretching vibration of ether oxide groups (C–O–C) in the region of 1050–1170 cm−1 of PEO and PEO-AlOC.
In addition, the supramolecular interactions between AlOC and PEO could break the crystallization tendency of PEO (Fig. 1c). We took the three-monomer molecular unit of PEO (CH2CH2OCH2CH2OCH2CH2O) as a model to perform theoretical calculations. As shown in Supplementary Fig. 1, there are mainly four types of sites on the nanocage involved in the interactions with PEO. Among them, Site I and Site II interact with PEO chains through a single aluminum molecular ring. The PEO chains are located in the pores formed by alternating up and down benzene rings and pyrazole rings. Site III and Site IV interact with PEO chains through the conjugated pore cavity formed by two vertically packed aluminum molecular rings. Moreover, all sites are generated through the interactions of C–H···π and C–H···O formed by the organic ligands on the aluminum molecular ring and PEO. It is worth noting that Site IV has the lowest adsorption energy of – 1.18 eV, which is the most likely interaction site. Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectra provide evidence for such interactions. As shown in Supplementary Fig. 2, when AlOC is mixed with PEO, two characteristic peaks of C–H in the aromatic nucleus also shifted from 722 cm−1 and 685 cm−1 to 728 cm−1 and 687 cm−1, respectively. In addition, the stretching vibration of ether oxide groups (C–O–C) in PEO at 1092 cm−1 will shift to 1097 cm−1 (Fig. 1d). The ether oxide groups (C–O–C) in PEO are the important parts of the chain folding structure in the PEO which usually induce the formation of crystalline phase of PEO37. On the other hand, the changes of Li+ and TFSI− in micro- and mesoscopic scales also influence the crystallization tendency of PEO. Obviously, such interactions will undoubtedly prevent the dense chain folding structure from the close packing between the segments to form the crystalline region.
Multi-scale characterization for Li-ions conduction network
Such supramolecular interactions have a great impact on the ion conduction network in the solid-state electrolytes. It is well known that the migration of ions in solid electrolytes is a multiscale process composed of mechanisms that manifest at different length scales, from the atomic scale up to the macroscopic scale. Importantly, the ionic conductivity of solid electrolytes is a function of all these mechanisms, which is typically measured on macroscopic samples through impedance spectroscopy measurements. The total macroscopic ionic conductivity is convoluted with each component, such as amorphous phases and crystalline phases. Given the complexity of ion conduction networks in solid-state electrolytes, the capabilities and limitations of single characterization technology could not provide comprehensive insights. In order to provide an accurate and reliable interpretation, we study ion conduction networks from atomic and nanoscale to macroscopic scales by multi-scale complementary characterization method.
As shown in Fig. 2a, b, high-resolution solid-state nuclear magnetic resonance is used to detect the local environments of lithium ions in atomic and nanoscale38. With the addition of AlOC, the peak has an obvious shift from 0.24 ppm to 0.37 ppm. It might suggest that in PEO-LiTFSI-AlOC, a less constrained coordination environment of Li-ions was built. Ionic migration, which is accompanied by a constant shift in the coordinating environment, benefits from such a phenomenon. In micro- and mesoscopic scales, the effect of such supramolecular interactions may be manifested in the variation of lithium-ion concentration. As shown in Fig. 2c and Fig. 2d, we employ Raman mapping technology to study the distribution of lithium ions in polymer electrolytes. When LiTFSI is dissolved into PEO polymer, there is a Raman shift of S–N–S in TFSI− anion at 740 cm−1. The linearity of the peak areas at this characteristic Raman shift can be used to determine the difference in the molar ratio of nLiTFSI: nEO across the whole PEO electrolyte (see Supplementary Discussion 2 for detailed information)39. Although the PEO polymer electrolyte was usually prepared with a specific molar ratio of nLiTFSI: nEO, for example, 1:15 in this work, the real Li-ion concentration distribution is not as expected uniformly among the whole electrolyte. The chemical potential difference between the crystalline and amorphous phases leads to a clear and distinct nLiTFSI: nEO heterogeneity among the polymer electrolytes. The statistical result based on the threshold segmentation method shows the average molar ratio of Li:EO in the crystalline phase and amorphous phase is calculated to be 1:16.4 and 1:12.2, respectively. This means that the lithium-ion concentration in the amorphous phase is about 1.3 times that in the crystalline phase. Raman mapping results reveal that the Li-ion concentration in both the amorphous phase and crystalline phase of PEO-LiTFSI-AlOC is changed obviously (Fig. 2d), the average nLiTFSI: nEO in the crystalline phase and amorphous phase is calculated to be 1:19.5 and 1:10.7, respectively (see Supplementary Discussion 2). In this regard, the lithium-ion concentration in the amorphous region is about 1.8 times that in the crystalline region. Compared with PEO-LiTFSI, there are greater concentrations of lithium ions in amorphous phases and lesser concentrations of lithium ions in crystalline phases for PEO-LiTFSI-AlOC. Considering the resistance of ion migration in amorphous phases and crystalline phases, this phenomenon is normally beneficial to the lithium-ion conductivity of polymer electrolytes.
a, b the 7Li MAS NMR spectra of PEO-LiTFSI (a) and PEO-LiTFSI-AlOC (b), respectively. c, d the Raman mapping characterization of the Li-ion concentration distribution in PEO-LiTFSI (c) and PEO-LiTFSI-AlOC (d), respectively; the left picture shows the 300 μm × 300 μm region at 10 × objective which overlaps the corresponding 10 × magnification optical image, and the right picture shows the small 100 μm × 100 μm region at 50 × objective. The color maps represent the molar ratio of Li:EO (More details can be found in Supplementary Discussion 2). The Li-ion conducting network of polymer electrolyte: e, f the volume renderings with the size of 300 μm × 300 μm × 90 μm of PEO-LiTFSI (e) and PEO-LiTFSI-AlOC (f); g, i the tomographic cross-section of PEO-LiTFSI (g) and PEO-LiTFSI-AlOC (i); (h, j) the proportion of crystalline and amorphous phases on the statistic of 150 XY plane ortho slices of PEO-LiTFSI (h) and PEO-LiTFSI-AlOC (j).
In addition, the information in the macroscopic scale is important to study the link between the material properties of polymer electrolyte and their ion conduction performance. We further used high spatial resolution and tunable propagation phase contrast XCT to visualize and quantify the microstructural heterogeneities of PEO-based polymer electrolytes at the macroscopic scale. The tunable propagation phase contrast enables the visualization of materials with low atomic numbers materials and the distinguishing of the poor absorption contrast polymers with different densities, which is different from the conventional absorption contrast-based X-rays absorption characterization technique (Supplementary Fig. 3)40. Thus, tiny changes in PEO polymer electrolyte produce actually considerable phase drifts. As shown in Fig. 2e, a 3D morphological distribution of crystallization and amorphous phases with a high sub-micro (600 nm) voxel resolution in a macroscopic size around 300 µm × 300 µm × 90 µm of PEO-LiTFSI polymer electrolyte can be obtained. The spatial proportion of the crystalline phase is ca. 66.7 % while the amorphous phase is ca. 33.3 %. As a result, the amorphous phase which is believed to be the more efficient Li-ion transportation channel only occupies a very small amount of area, which was mostly surrounded by the large crystalline phases (Fig. 2g). The large size of the crystalline phases will result in a very low proportion of amorphous phases (< 40 %) based on the statistics of all ortho slices cross sections (Fig. 2h). This is unable to form a complete and continuous 3D amorphous network structure at such a low proportion of amorphous phases (Supplementary Fig. 4).
There are significant changes in the spatial proportion of the crystalline region and the amorphous region for the PEO-based Li-ion conduction network (Fig. 2f and Supplementary Fig. 5). The spatial proportion of the amorphous phase is around 64.6 % and it is 35.4 % for the crystalline phase in PEO-LiTFSI-AlOC. Compared with the above XCT results of PEO-LiTFSI (Fig. 2e), the spatial proportion of the amorphous phase in PEO-LiTFSI-AlOC (64.6 %) is much higher than the one of PEO-LiTFSI (33.3 %). The continuity of amorphous phases in PEO-LiTFSI-AlOC has been significantly improved. In the tomographic cross-section (Fig. 2i), it is difficult to find any isolated amorphous phase, and most of the crystalline phases are independent small-sized spherulites. The particle size of the crystalline phase is also reduced to around ~ 20–40 µm (Supplementary Figs. 7, 8), which is much smaller than the ones in the PEO-LiTFSI. Thus, due to a much lower spatial proportion and smaller particle size of the crystalline phase, the proportion of the amorphous phase retains a high proportion of > 55 % based on the statistics of all cross sections, and even some planes accounted for as high as 68.2 % (Fig. 2j).
For a multiscale ion conduction process, the information from several distinct techniques is inevitably interrelated. The internal connections need further excavated to analyze the link between the material properties of polymer electrolyte and their ion conduction performance (Supplementary Table. 1). Correlating lithium ion concentration and macroscopic component, we could calculate that there is as low as 39.4 % of the lithium ions in total for Li-ion conducting and transporting within the amorphous phases. The mobility of lithium-ion in amorphous phases is higher than lithium-ion in crystalline phases. Moreover, the isolated amorphous region with poor space connectivity, as discussed above, will not contribute the effective Li-ion conduction. Besides, the strong interaction between EO unites and Li-ion in conventional PEO-LiTFSI electrolytes will undoubtedly increase the dissolving of Li-ion salts. However, such strong Li-ions coordination will also retard the motion of Li-ion among the EO units in the polymer electrolytes10. As a result, conventional PEO-LiTFSI electrolytes exhibit as low as 10−6 S cm−1 conductivity at near room temperature. In contrast, the introduction of AlOC reconstructed the ion conduction network from multiple scales. The interactions at the atomic and molecular levels bring about a significant influence on the macroscopic polymer electrolyte to form the continuous amorphous phase structure. The statistical result also shows there is around 76.7 % of the lithium ions in total for Li-ion conducting and transporting via the amorphous phase network (it exceeds the proportion of 39.4 % in PEO-LiTFSI). These results indicate an effective and continuous Li-ion-rich conducting network is successfully built in the bulk of PEO-LiTFSI-AlOC. In addition, the adsorption of anions and segments on AlOC may reduce the resistance of the transformation of Li-ions coordination, which is beneficial for ion conduction.
The Li-ions transport dynamics and conductivity
Due to the reconstructing in the 3D Li-ion transportation network, the Li-ions in PEO-LiTFSI and PEO-LiTFSI-AlOC deliver distinguish conduction ability when coupled with the segmental relaxation of polymers41. Ion conduction in the crystalline phase with frozen segmental relaxation should be more sluggish than in amorphous phases with rapid segmental relaxation. The amorphous phases with ca. 33.3 % were divided into several isolated amorphous phases by crystalline phase with ca. 66.7 % in PEO-LiTFSI. In this condition, the limitation of the crystalline phase to the diffusion of Li-ions will cause a change in Li-ion transport kinetics. It appears as two distinct Arrhenius plots (Fig. 3f), with an activation energy of 1.21 eV and 0.42 eV below and higher than the melting temperature point of 59.6 °Ϲ (Supplementary Fig. 9), respectively. Due to the high activation energy below Tm, the ionic conductivity of PEO-LiTFSI shows a cliff-like decline, which has a very low ionic conductivity of 10−6 S cm−1 at near room temperature (Supplementary Table. 2). Due to the ability to reconstruct and enhance the ionic conduction network by AlOC, an effective and continuous Li-ion-rich conducting network can be built with a much higher spatial proportion of amorphous regions (64.6 %) and much higher available Li-ions concentrations in the amorphous Li-ion conducting polymer network. This effectively reduces the diffusive lengthscales of Li-ions in the crystalline phase. Thus, the Li-ion conductivity σ follows an Arrhenius equation with a lower activation energy of 0.42 eV in the whole temperature range, which was equivalent to that of PEO-LiTFSI in high temperature. As a result, the PEO-LiTFSI-AlOC superionic PEO conductor exhibited a molten-like Li-ion conduction behavior among the whole temperature range and delivered an ionic conductivity approaching to 1.87 × 10−4 S cm−1 at 35 °Ϲ. The Bruce–Vincent method d.c. polarization measurements also show the change in the lithium-ion transference number. PEO-LiTFSI only shows a low lithium ion transference number of 0.0931, which is much lower than 0.42 of PEO-LiTFSI-AlOC (Supplementary Fig. 10 and Supplementary Table. 3). It indicates the ionic current is carried predominantly by the Li-ion in PEO-LiTFSI-AlOC rather than its counter-ion.
The ionic conductivity (σ) versus temperature (T) of PEO-LiTFSI and PEO-LiTFSI-AlOC, the error bars correspond to the standard deviation from the average \(\log (\sigma )\) obtained by measuring at least five individual experiments at each temperature point. The specific fitting equation of Arrhenius plots and the calculation of activation energy can be found in Methods.
The Li plating/stripping behavior in Li symmetric cells
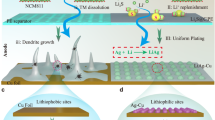
Such a 3D Li-ion conducting network with high connectivity also brings about an obvious influence on the Li electrochemical plating and stripping behaviors in symmetric Li cells. Due to the poor Li-ion conduction behavior, the Li|PEO-LiTFSI|Li symmetric cell has a large overpotential of 414.8 mV in the preliminary stage at 50 μA cm−2 at 35 °Ϲ (Supplementary Fig. 11). The overpotential will gradually increase to 570 mV and finally sudden short circuit at 366 h due to the Li dendrite penetration. However, there is no obvious voltage oscillation of the Li|PEO-LiTFSI-AlOC|Li symmetric cell at a current density of 50 μA cm−2 for an ultra-long duration of 2000 h (Fig. 4a). In addition, the Li plating/stripping behavior is highly stable when the current density increases from 25 μA cm−2 to 100 μA cm−2 (Fig. 4b). When the current density returned to 25 μA cm−2, the overpotential is recovered to ~ 33 mV and the Li electrochemical plating and stripping can be further operated steadily over 1700 h without short circuit (Supplementary Fig. 12a). PEO-LiTFSI-AlOC superionic PEO conductor even deliver an enhanced rate performance of Li plating/stripping at 50 °Ϲ (still below the melting point). Long-term cycle test confirms that PEO-LiTFSI-AlOC exhibits extremely stable Li plating/stripping behavior at a current density of 50 μA cm−2 for 2000 h with a stable overpotential of ~39.9 mV (Fig. 4c). The symmetric Li cell also shows stable Li plating/stripping behavior for 600 h with the overpotential of ~215.7 mV even at a higher current density of 250 μA cm−2 (Supplementary Fig. 12b). With the current density increasing from 50 to 400 μA cm−2, the overpotential increases from 66.6 mV to 394.5 mV (Fig. 4d). In contrast, under the current density of 250 μA cm−2, the Li|PEO-LiTFSI|Li symmetric cell shows a significant increase in overpotential at initial stage and sudden short circuit after 9 h (Supplementary Fig. 12c). This is because the insufficient Li-ion conductivity of PEO-LiTFSI which will result in the severe Li dendrite in the symmetric cell.
The long-term cycling performance of Li|PEO-LiTFSI-AlOC|Li symmetric cells at the current density of 50 μA cm−2 at 35 °Ϲ (a) and 50 °Ϲ (c). The inset figures show the voltage curves of the cycle at 1st, 500th, and 1000th. The cycling performance of Li|PEO-LiTFSI-AlOC|Li symmetric cells under different current densities at 35 °Ϲ (b) and 50 °Ϲ (d).
The performance of all-solid-state Li | LiFePO4 full batteries
Unlike most of the PEO-based, all solid-state rechargeable batteries require high temperatures (usually higher than 60 °Ϲ) to allow an acceptable battery performance, the Li|PEO-LiTFSI-AlOC|LiFePO4 full batteries exhibit a good performance below its melting point temperature of 56.0 °Ϲ. For example, it exhibits typical discharge plateaus at 3.35 V and charge plateaus at 3.50 V during the Galvanostatic charging–discharging under 35 °Ϲ. High capacities of 161.8, 155.7, 142.2, and 127.5 mAh g−1 can be obtained at various current densities of 25 μA cm−2, 50 μA cm−2, 75 μA cm−2, and 100 μA cm−2, respectively (Fig. 5a). And the capacity can be recovered to 158.0 mAh g−1 when the current density was returned to 25 μA cm−2 (Supplementary Fig. 13). Particularly, the overpotentials are only 153 mV at 25 μA cm−2 and 226 mV at 50 μA cm−2, respectively. These results indicate a superior capacity retention at various current densities can be retained. Moreover, the as-built ASSBs show a stable battery cycling performance at 35 °Ϲ (Fig. 5e). There is no obvious capacity fading over 200 cycles and the specific capacities can be retained around 156 mAh g−1 at 25 μA cm−2 with an average coulombic efficiency (CE) higher than 99.99 %. Whereas Li|PEO-LiTFSI |LiFePO4 only delivers a discharge-specific capacity of ~ 120 mAh g−1, and the CE dramatically decreases after 40 cycles (Supplementary Fig. 14a). When the operating temperature increases to 50 °Ϲ, high and reversible capacity can be further enhanced with much lower overpotential even at a much higher current density (Fig. 5b). The cell maintains very low overpotentials of 113.8 mV at 100 μA cm−2 and 179.8 mV at 200 μA cm−2, respectively. Even at 400 μA cm−2, it delivers a reversible specific capacity of 139.0 mAh g−1 with an overpotential of 299 mV. In the long-term cycling at 250 μA cm−2, the cell also maintains a high discharge capacity of ~ 147 mAh g−1 and sustains > 96 % capacity over 200 cycles (Fig. 5e). In contrast, Li|PEO-LiTFSI|LiFePO4 has poor performance even at 50 °Ϲ with a discharge capacity of ~ 70 mAh g−1 at 250 μA cm−2 (Supplementary Fig. 14b).
a, b the galvanostatic charge-discharge curves of Li|PEO-LiTFSI-AlOC|LiFePO4 as a function of current density at 35 °Ϲ (a) and 50 °Ϲ (b), respectively. c, d the galvanostatic charge-discharge curves as a function of cathode mass loading under 10 μA cm−2 at 35 °Ϲ (c) and 25 μA cm−2 at 50 °Ϲ (d) respectively; (e) the cycling performances of Li|PEO-LiTFSI-AlOC|LiFePO4 under different areal current densities and operating temperatures. f the battery performances comparison of this work and the reported polymer-based all-solid-state Li batteries (25–40 °Ϲ: green, 41–59 °Ϲ: blue, 60–69 °Ϲ: dark blue and higher than 70 °Ϲ: black). See Supplementary Table. 4 for details. g the galvanostatic charge-discharge curves of the pouch cell with LFP loading of 7.15 mg cm−2 at the current density of 10 μA cm−2.
In all solid-state polymer rechargeable batteries, lithium ions can only be transported across the network of the polymer electrolyte via solid-solid interfaces. Thus, a sufficient 3D Li-ion conduction network across the whole electrode is crucial to allow the feasible intercalation/deintercalation of Li-ion in LiFePO4 cathode at a high mass loading. With the increase of the cathode mass loading, the electrode thickness will be increased from 43 μm at 4.1 mg cm−2 to 84 μm at 16.1 mg cm−2 (detailed see Methods, Supplementary Fig. 15 and Supplementary Fig. 16). At the operating temperature of 35 °C, Li|PEO-LiTFSI-AlOC|LiFePO4 full rechargeable batteries can still display high specific capacities of 157.6, 146.2, and 143.4 mAh g−1 with mass loadings of 2.0, 5.2, and 11.0 mg cm−2, respectively (Fig. 5c). And it also delivers a high specific capacity of over 150 mAh g−1 with an overpotential lower than 130.0 mV even at a very high mass loading of 16.8 mg cm−2 at 50 °Ϲ (Fig. 5d). The reversible specific capacities follow an almost linear relationship with the areal capacities at various mass loadings (Supplementary Fig. 17). And a high areal capacity of 1.58 mAh cm−2 and 2.53 mAh cm−2 can be achieved at 35 °Ϲ and 50 °Ϲ, respectively. These performances are advanced in the state-of-art of present polymer-based ASSBs (Supplementary Table. 4)5,6,7,8,9,12,17,24,25,28,42,43,44,45,46,47,48,49. As shown in Fig. 5f, it is still a challenge to make the areal capacity more than 1 mAh cm−2 at the operating temperatures of 25–40 °Ϲ. In order to exceed the areal capacity higher than 2 mAh cm−2, an operating temperature higher than the melting point of polymer electrolyte is usually required. Furthermore, we assemble a single-electrode pouch cell based on the single-side-coated LFP composite cathode (active area of 56 × 43 mm2), and the mass loading of LFP is 7.15 mg cm−2. As shown in Fig. 5g, the pouch cell delivers a reversible capacity of 163.7 mAh g−1, meaning a high output capacity of 1.17 mAh cm−2. Such greatly improved battery rate performance at various mass loading in this work is due to the enhancement and reconstruction of the continuous amorphous ionic conduction network of PEO-LiTFSI-AlOC.
In summary, we introduce a kind of aluminum-oxo rings to effectively improve the Li-ion conduction network of PEO-based electrolytes via the supramolecular interactions, including the interactions of C-H···π and C-H···O between AlOC and PEO chains, host–guest interactions between AlOC and LiTFSI. The multi-scale complementary characterization technologies are adapted for the detailed study of ion conduction networks in polymer electrolytes. Lithium ions with different local environments in atomic and nanoscale are quantified by solid-state nuclear magnetic resonance. Raman spectroscopy mapping techniques and high spatial resolution and tunable propagation phase contrast XCT are employed to visualize and quantify microstructural heterogeneities of PEO-based polymer electrolytes. According to multi-scale characterization analysis, we also reveal that the conventional PEO-based electrolyte has up to ~ 60.6 % of lithium ions shackled within the crystalline phase. The amorphous Li-ion conducting polymer network with only 39.4 % available Li-ions is disconnected by the crystalline phase of PEO. In contrast, the PEO-LiTFSI-AlOC superionic PEO conductor has an effective and continuous Li-ion-rich conducting network with a much higher spatial proportion of amorphous regions of 64.6 % than that of 33.3 % in conventional PEO electrolyte. The available Li-ions in the amorphous Li-ion conducting polymer network are also increased to 76.7 %. Such reconstruction of the 3D ion conduction network brings in molten-like Li-ion conduction behavior with an ionic conductivity of 1.87 × 10−4 S cm−1 at 35 °Ϲ and a stable Li electrochemical plating/stripping performance over 2000 h. The as-built Li|PEO-LiTFSI-AlOC|LiFePO4 full batteries show a high rate performance and long cycling performance with capacity retention of more than 90 % and an average CE higher than 99.9 % over 200 cyclings and further enable a high-loading LiFePO4 cathode of 16.8 mg cm−2 with a specific capacity of 150 mAh cm−2.
Methods
Preparation of solid electrolyte
For synthesis of AlOC, a mixture of aluminum isopropoxide (204 mg, 1 mmol, Aladdin Chemical Reagent Shanghai), 4-methylpyrazole (4 g, 48.72 mmol, Aladdin Chemical Reagent Shanghai), benzoic acid (122 mg, 1 mmol, Aladdin Chemical Reagent Shanghai), piperazine (86.14 mg, 1 mmol, Aladdin Chemical Reagent Shanghai) and DMF (2 mL, Sinopharm Chemical Reagent Beijing) was sealed in a 20 mL vial and transferred to a preheated oven at 100 °Ϲ for 3 days. When cooled to room temperature, octahedron colorless crystals were obtained. (yield: 56 % based on Al(OiPr)3). The crystals were obtained after rinsed with DMF and preserved under a sealed and dry environment.
To prepare solid polymer electrolytes, PEO (MW = 600000, Aladdin Chemical Reagent Shanghai) and LiTFSI (DoDoChem) were dissolved in anhydrous acetonitrile (Aladdin Chemical Reagent Shanghai). The Li to EO molar ratio was 1:15. The mixture was continuously stirred at 600 r.p.m. at 50 °Ϲ for 24 h to ensure the formation of a homogeneous solution. Pure PEO-LiTFSI films were prepared by doctor blade casting followed by drying at 40 °Ϲ for 24 h and drying in a vacuum oven at 60 °Ϲ for 24 h, then standing in an Ar-filled glove box at room temperature for at least 48 h. PEO-LiTFSI-AlOC superionic PEO conductors were obtained in a facile two-step process. First, AlOC was ultrasonically dispersed in acetonitrile for at least 2 h to form a homogeneous AlOC dispersion. Under the conditions with 600 r.p.m and 50 °Ϲ, the AlOC dispersion was slowly added to the as-prepared PEO-LiTFSI acetonitrile solution. The mass fraction of AlOC is 5 %. Then, the PEO-LiTFSI-AlOC film underwent the same drying process as the regular PEO-LiTFSI film. All the procedures sensitive to moisture and oxygen took place in an Ar-filled glove box.
Raman spectroscopy characterization and data analysis
The polymer electrolyte was clamped in two slides, and the edge of the slide was sealed with tape to prevent the solid electrolyte from absorbing water during the test. The Raman spectroscopy was collected with a confocal Raman microscope (HORIBA LabRAM HR Evolution) with an excitation wavelength of 532 nm. In order to get better resolution, the laser power was set to be more than 20 mW at 10 × objective and 5 mW at 50 × objective. The spatial resolution is ~ 3 µm for a 10 × objective lens and ~ 1.5 µm for a 50 × objective lens, respectively. To obtain the Raman mapping images, a stage was used to move the sample with a step size of 3 µm for a 10 × objective lens and 1.5 µm for a 50 × objective lens, and a Raman spectrum was recorded at every point.
The pre-processing of the Raman spectrum included two steps: first, the background of the spectra is removed by the auto-adaptive background subtraction method. Second, all spectra are normalized to the maximum Raman intensity in the range 1450–1500 cm−1, which is assigned to the CH2 bending vibrations of PEO (Supplementary Discussion 2).
X-ray computed tomography and 3D image data analysis
The polymer electrolyte was scanned using a lab-based X-ray nano-CT system (Zeiss Xradia Versa 610 X-ray computed tomography microscope, Carl Zeiss, CA, USA) at an isotropic voxel size of 597 nm and a field of view of Φ590 μm × 590 μm. X-ray beams with a voltage and current of 70 kV and 85 µA were used and ~ 950 sequential projections with an exposure time of 1 s were collected over 360° rotation. The corrected, aligned radiographs were reconstructed using standard, parallel beam, filtered-back projection algorithms, implemented in the Zeiss Scout and Scan software package (Carl Zeiss, CA, USA). The reconstructed 3D volume of the polymer electrolyte was imported into the commercial software package AVIZO for microstructural characterization. Firstly, we used the watershed algorithm method to initially identify the phase boundary. Secondly, the regions with the maximum and minimum gray values are selected as the seed regions of the crystalline and amorphous phases for dilation and erosion, respectively. Then, in the process of dilation, amorphous phases or crystalline phases will inevitably intersect with the phase boundary. The Boolean operation is used to deduct the intersection until the crystalline phases and the amorphous phases are filled in all regions except the phase boundary. Finally, the phase boundary is divided into two parts by the watershed algorithm. The area with high gray value is added to crystalline phases and the area with low gray value is added to amorphous phases.
Other characterization
Fourier transform infrared spectroscopy spectra (FTIR) were measured using a Bruker Vertex 70 V spectrometer in an attenuated total reflectance setup under vacuum conditions (The measured results are shown in Supplementary Fig. 22 and Supplementary Table. 6). Solid-State Nuclear Magnetic Resonance (Bruker AVANCE NEO 600) was applied to detect the local environments of ions of solid polymer electrolyte (Fig. 2a, band Supplementary Fig. 27). Differential scanning calorimetry curves were obtained using a − 70 ~ 150 °Ϲ under a nitrogen atmosphere at a heating and cooling rate of 10 °Ϲ min−1 (Supplementary Figs. 9, 26 and Supplementary Table 7). The morphology of solid electrolytes was investigated by a field emission scanning electron microscope (Zeiss GeminiSEM 500). (Supplementary Fig. 7 and Supplementary Figs. 15, 24).
The symmetric cell performance measurements
Lithium metal foils with a thickness of 1 mm and a diameter of 15.6 mm. In order to explore the reversibility of the Li electrochemical plating and stripping behaviors using polymer electrolyte, the symmetric Li–Li symmetric cells were tested by a galvanostatic cycling measurement with charging and discharging for 1 h. To further measure the interface stability of solid polymer electrolyte and Li metal electrode, different current densities of 25 μA cm−2, 50 μA cm−2, 75 μA cm−2, 100 μA cm−2 were applied at 35 °Ϲ and 50 μA cm−2, 100 μA cm−2, 150 μA cm−2, 200 μA cm−2, 250 μA cm−2, 300 μA cm−2, 350 μA cm−2, 400 μA cm−2 were applied at 50 °Ϲ.
LiFePO4 cathode electrode preparation and performance characterization
LiFePO4 composite cathodes were prepared using a slurry casting technique. Active material (LiFePO4, LFP), super P carbon as a conductive additive, polyvinylidene fluoride (PVDF), and PEO-LiTFSI-AlOC were dispersed in N-methylpyrrolidone (NMP) with a weight ratio of 8:1:0.5:0.5 and ball milled to form a homogeneous slurry. Then, the slurry was cast on a carbon-coated aluminum current collector and dried under vacuum at 80 °Ϲ for 24 h. The active loading density was 1-2 mg cm−2.
When the electrode thickness increases, the slow ion transport significantly increases the diversity of the particle environment, which poses significant hurdles to the charge-transfer kinetics, the active materials utilization, and the long cycling stability for solid-state batteries. Thus, it is necessary to increase the solid electrolyte content for constructing continuous Li+ transport networks in solid-state cathodes. The cathode consists of 70 wt% LFP, 10 wt% Super P, 15 wt% PEO-LiTFSI-AlOC and 5 wt% PVDF. The mass loading of active material is 1~ 18 mg cm−2.
The theoretical capacity of LFP is 170 mAh g−1. The compacted density of all LiFePO4 composite cathodes is around 2 g cm−3.
The full cell performance measurements
All solid-state half-cells were assembled as CR2032-type coin cells using LiFePO4 as the cathode and lithium metal foil as an anode. Galvanostatic charge/discharge cycling and rate performance tests of all solid-state half-cell batteries were conducted on a battery test system (Neware, CT-4008). The cut-off voltages were 2.4 V − 4.0 V. All temperature-controlled experiments were tested inside an environmental chamber with 35 °Ϲ or 50 °Ϲ (Boxun, SPX-150B III) using a precision thermometer (± 0.5 °Ϲ). The batteries stand in the environmental chamber for at least 3 h prior to testing. In the long-term cycling measurement of Li|PEO-LiTFSI-AlOC|LiFePO4, the coulombic efficiency for each cycle was collected to calculate the average coulombic efficiency.
The pouch cell assembly and measurements
For the cathode side, the cathode consists of 70 wt% LFP, 10 wt% Super P, 15 wt% PEO-LiTFSI-AlOC and 5 wt% PVDF. The mass loading of active material is ~ 7 mg cm−2. The LFP electrode sheets were cut into a predetermined size. The effective area was 24.08 cm2 (5.6 cm × 4.3 cm). For polymer electrolyte, the PEO-LiTFSI-AlOC membrane was cut into a rectangle of 6.0 cm × 5.0 cm. The thickness of the polymeric electrolyte was about ~ 100 μm. For the anode side, the lithium foil with a thickness of 250 μm was physically pressed in Cu mesh (100 μm) as the anode. The anode was cut into a rectangle of 5.8 × 4.5 cm. The pouch cells were assembled and sealed in a laminated aluminum film bag. The pouch cell assembly and electrode cutting take place in a drying room with a dew point temperature of − 40 °Ϲ.
The assembled pouch cell was put into a fixture. The fixture consists of two smooth stainless steel plates and four long screws, which apply a pressure of ~ 0.3 MPa to the pouch cell. Before the test, the fixture and pouch cell were put in an environmental chamber with 60 °Ϲ for 10 h to form a stable interface between the polymer electrolyte. The galvanostatic charge-discharge curve of the pouch cell was measured at 10 μA cm−1 and 50 °Ϲ.
Fundamental electrochemistry measurements
CR2025-type coin cell was applied to assemble the symmetric cells. Stainless steel sheets (SS) with a thickness of 1 mm and a diameter of 15.8 mm. Lithium metal foils with a thickness of 1 mm and a diameter of 15.6 mm. All batteries are assembled in an Ar-filled glove box with water and oxygen below 0.1 ppm.
EIS of polymer electrolyte was tested on an impedance analyzer (Solartron1260) with an AC amplitude of 5 mV and a frequency range of 1 MHz to 1 Hz at an open-circuit voltage. The ionic conductivity of the polymer electrolyte was measured using EIS with SS|electrolyte|SS symmetric cells at different temperatures. Before testing, the SS symmetric cells were held at the required temperature for one hour. The ionic conductivity (σ) was calculated according to:
where L is the thickness of the polymer electrolyte, R is the bulk resistance and S is the area. Five parallel experiments were carried out in PEO-LiTFSI and PEO-LiTFSI-AlOC. Detailed information is shown in Supplementary Figs. 18, 19, and Supplementary Table. 5.
The activation energy was measured from the Arrhenius equation:
where A is the exponential factor, kB is the Boltzmann constant, T is the Kelvin temperature, σ is the ionic conductivity, and Ea is the activation energy.
The Li+ transference numbers (tLi) in PEO-LiTFSI and PEO-LiTFSI-AlOC were measured by using the Bruce–Vincent method. d.c. polarization measurements were conducted with a potential of ∆V = 20 mV in Li|PEO-LiTFSI|Li and Li|PEO-LiTFSI-AlOC|Li cells until the current reached a steady state, and corresponding EIS measurements were collected before and after the d.c. polarization (Supplementary Fig. 10).
tLi was calculated according to:
where ΔV is the applied potential, I0 is the initial current, R0 is the initial resistance, Iss is the steady-state current, and Rss is the steady-state resistance. The measured results of these parameters are listed in the Supplementary Table. 3.
Theoretical simulation
The simulation of host–guest interactions between LiTFSI and AlOC
The initial structure of the adsorption model is derived by abstracting the structure of the cluster from the crystal structure obtained during the experiment and combining it with the guest LiTFSI. Optimization of the structure of the adsorption model by using the xTB program. Clarify the interaction between cluster and guest by using the QM/GFN2-xTB method and the ORCA program. The hybrid functional TPSSh and the basis set def2-SVP are used in the QM region. Calculate the Interaction Region Index (IRI) of the capture sites by using the Multiwfn program. Detailed information can be found in the Supplementary Discussion 1.
Data availability
The authors declare that all the relevant data within this paper and its Supplementary Information file are available from the corresponding author upon request.
References
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Zheng, Y. et al. A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures. Chem. Soc. Rev. 49, 8790–8839 (2020).
Chen, R., Li, Q., Yu, X., Chen, L. & Li, H. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2020).
Dixit, M. B. et al. Nanoscale mapping of extrinsic interfaces in hybrid solid electrolytes. Joule 4, 207–221 (2020).
Zhang, K. et al. 8.5 µm-thick flexible-rigid hybrid solid-electrolyte/lithium integration for air-stable and interface-compatible all-solid-state lithium metal batteries. Adv. Energy Mater. 12, 2200368 (2022).
Xu, H. et al. Phase-changeable dynamic conformal electrode/electrolyte interlayer enabling pressure-independent solid-state lithium metal batteries. Adv. Mater. 35, 2212111 (2023).
Ding, P. et al. Molecular self-assembled ether-based polyrotaxane solid electrolyte for lithium metal batteries. J. Am. Chem. Soc. 145, 1548–1556 (2023).
Yang, L. et al. Heterogeneous nanodomain electrolytes for ultra-long-life all-solid-state lithium-metal batteries. Adv. Funct. Mater. 32, 2204778 (2022).
Yang, X. et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: main chain or terminal –OH group? Energy Environ. Sci. 13, 1318–1325 (2020).
Molinari, N. et al. Effect of salt concentration on ion clustering and transport in polymer solid electrolytes: A molecular dynamics study of PEO−LiTFSI. Chem. Mater. 30, 6298–6306 (2018).
Qian, L. X. et al. Anion π−π stacking for improved lithium transport in polymerelectrolytes. J. Am. Chem. Soc. 144, 9806–9816 (2022).
Wan, J. et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 14, 705–711 (2019).
Zhou, D., Shanmukaraj, D., Tkacheva, A., Armand, M. & Wang, G. Polymer electrolytes for lithium-based batteries: advances and prospects. Chem 5, 2326–2352 (2019).
Xu, S. et al. Strain-induced multiscale structural evolutions of crystallized polymers: From fundamental studies to recent progresses. Prog. Polym. Sci. 140, 101676 (2023).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013).
Hashimoto, K. et al. Strain-induced crystallization and phase separation used for fabricating a tough and stiff slide-ring solid polymer electrolyte. Sci. Adv. 9, eadi8505 (2023).
Liu, M. et al. Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat. Nanotechnol. 17, 959–967 (2022).
Wang, B. H., Xia, T., Chen, Q. & Yao, Y. F. Probing the dynamics of Li+ ions on the crystal surface: a solid-state NMR study. Polymers 12, 391 (2020).
Cheng, Z. et al. Revealing the impact of space-charge layers on the Li-ion transport in all-solid-state batteries. Joule 4, 1311–1323 (2020).
Scharf, J. et al. Bridging nano- and microscale X-ray tomography for battery research by leveraging artificial intelligence. Nat. Nanotechnol. 17, 446–459 (2022).
Li, J. et al. Dynamics of particle network in composite battery cathodes. Science 376, 517–521 (2022).
Lewis, J. A. et al. Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nat. Mater. 20, 503–510 (2021).
Ning, Z. et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021).
Su, Y. et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 13, 4181 (2022).
Zhang, M. et al. Constructing the high-areal-capacity, solid-state Li polymer battery via the multiscale ion transport pathway design. Mater. Today 56, 53–65 (2022).
Shi, Y. Y. et al. Atomic-scale Al2O3 modified PEO-based composite polymer electrolyte for durable solid-state Li-S. J. Electroanal. Chem. 881, 114916 (2021).
Lin, C. W., Hung, C. L., Venkateswarlu, M. & Hwang, B. J. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 146, 397–401 (2005).
Lin, D. et al. A Silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 30, 1802661 (2018).
Zheng, J., Tang, M. & Hu, Y. Y. Lithium ion pathway within Li7La3Zr2O12-Polyethylene oxide composite electrolytes. Angew. Chem. Int. Ed. 55, 12538–12542 (2016).
Gupta, A. & Sakamoto, J. Controlling ionic transport through the PEO-LiTFSI/LLZTO interface. Electrochem. Soc. Interface 28, 63–69 (2019).
Hou, H. Y. et al. Anion-immobilized polymer electrolyte achieved by cationic metal-organic framework filler for dendrite-free solid-state batteries. Energy Stor. Mater. 18, 59–67 (2019).
Chen, J. J., Symes, M. D. & Cronin, L. Highly reduced and protonated aqueous solutions of [P2W18O62]6- for on-demand hydrogen generation and energy storage. Nat. Chem. 10, 1042–1047 (2018).
Busche, C. et al. Design and fabrication of memory devices based on nanoscale polyoxometalate clusters. Nature 515, 545–549 (2014).
Martin-Sabi, M. et al. Redox tuning the Weakley-type polyoxometalate archetype for the oxygen evolution reaction. Nat. Catal. 1, 208–213 (2018).
Amthor, S. et al. A photosensitizer–polyoxometalate dyad that enables the decoupling of light and dark reactions for delayed on-demand solar hydrogen production. Nat. Chem. 14, 321–327 (2022).
Yao, S., Fang, W. H., Sun, Y., Wang, S. T. & Zhang, J. Mesoporous assembly of aluminum molecular rings for iodine capture. J. Am. Chem. Soc. 143, 2325–2330 (2021).
Dingler, C., Dirnberger, K. & Ludwigs, S. Semiconducting polymer spherulites-from fundamentals to polymer electronics. Macromol. Rapid Commun. 40, 1800601 (2019).
Wu, N. et al. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte. Angew. Chem. Int. Ed. 59, 4131–4137 (2020).
Edman, L. Ion association and ion solvation effects at the crystalline−amorphous phase transition in PEO−LiTFSI. J. Phys. Chem. B 104, 7254–7258 (2000).
Falch, K. V. et al. Zernike phase contrast in high-energy x-ray transmission microscopy based on refractive optics. Ultramicroscopy 184, 267–273 (2018).
Bocharova, V. & Sokolov, A. P. Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 53, 4141–4157 (2020).
Yang, X. et al. High-areal-capacity all-solid-state lithium batteries enabled by rational design of fast ion transport channels in vertically-aligned composite polymer electrodes. Nano Energy 61, 567–575 (2019).
Jiang, T. et al. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv. Energy Mater. 10, 1903376 (2020).
Bi, Z. et al. Cathode supported solid lithium batteries enabling high energy density and stable cyclability. Energy Stor. Mater 35, 512–519 (2021).
Chen, S. et al. All-solid-state batteries with a limited lithium metal anode at room temperature using a garnet-based electrolyte. Adv. Mater. 33, 2002325 (2021).
Lin, Y. K. et al. A high-capacity, long-cycling all-solid-state lithium battery enabled by integrated cathode/ultrathin solid electrolyte. Adv. Energy Mater. 11, 2101612 (2021).
Paolella, A. et al. Enabling high-performance NASICON-based solid-state lithium metal batteries towards practical conditions. Adv. Funct. Mater. 31, 2102765 (2021).
Pazhaniswamy, S., Joshi, S. A., Hou, H., Parameswaran, A. K. & Agarwal, S. Hybrid polymer electrolyte encased cathode particles interface-based core-shell structure for high-performance room temperature all-solid-state batteries. Adv. Energy Mater. 13, 2202981 (2022).
Nie, Y. H. et al. Tailoring vertically aligned inorganic-polymer nanocomposites with abundant Lewis acid sites for ultra-stable solid-state lithium metal batteries. Adv. Energy Mater. 13, 202204218 (2023).
Acknowledgements
We appreciate the useful discussion with Prof. Zhong-Qun Tian and Prof. Bin Ren (Xiamen University). J.-J. C., C.-D.F., Y.H., P.-F.S., and K.L. acknowledge financial support from the National Natural Science Foundation of China (NSFC, 22393901, 22021001, 22272143), National Key Research and Development Program (2021YFA1502300), the Fundamental Research Funds for the Central Universities (20720220009), and the Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM, RD2023020801). W.-H.F., Y.-F.S., S.-Y.Y., and M.-Y. Z. acknowledge financial support from the National Natural Science Foundation of China (NSFC, 22371278).
Author information
Authors and Affiliations
Contributions
J.-J.C. conceived the original ideas and concept and directed the project. J.-J.C., together with W.-H.F., Y.H., and C.-D.F. expanded the hypothesis, planned experiments, and wrote the paper. Y.H., C.-D.F., and Y.-F.S. designed the experiments and conducted the material preparation and characterization. Y.H., C.-D.F., and P.-F.S. collected and analyzed XCT data. Y.H., C.-D.F., and K.L. collected and analyzed Raman mapping data. Y.H., C.-D.F., P.-F.S., and K.L. carried out electrochemical tests. M.-Y. Z., S.-Y.Y., Y.-F.S., and W.-H.F. carried out AIMD simulations. Y.H., C.-D.F., P.-F.S., and K.L. participated in data analysis and discussion. Y.H. and C.-D.F. prepared the LFP cathode and carried out electrochemical tests. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sang Uck Lee and the other anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, CD., Huang, Y., Sun, YF. et al. Revealing and reconstructing the 3D Li-ion transportation network for superionic poly(ethylene) oxide conductor. Nat Commun 15, 6781 (2024). https://doi.org/10.1038/s41467-024-51191-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51191-2