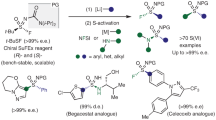
Abstract
Efforts aimed at enriching the chemical and structural diversity of small molecules have invigorated synthetic exploration in the last two decades. Spatially defined molecular functionality serves as the foundation to construct unique chemical space to further advance discovery science. The chiral SuFEx reagent t-BuSF provides a modular platform for the stereocontrolled bifunctionalization of sulfur. Here we report a third functional feature of t-BuSF enabled by carbamoyl torsional strain-release that further expands the S(IV) and S(VI) chemical space accessible as showcased in over seventy examples, multiple applications in medicinal chemistry, organocatalysis, and diversity-oriented synthesis. The methods presented herein allow for rapid asymmetric diversification around a stereodefined sulfur center with readily available building blocks, improving upon the current state-of-the-art for sulfinyl and sulfonimidoyl synthesis.
Similar content being viewed by others
Introduction
Sulfur in its range of oxidation states is ubiquitous in nature and the modern industrial world. From the organosulfur compounds found in essential biomolecules such as amino acids (cysteine and methionine) and vitamins (biotin), to life saving antibiotics (penicillin) and antidiabetic medicines (glibenclamide), sulfur’s rich chemical and structural diversity has profound impacts on all living organisms1,2,3. Sulfonylureas, such as glibenclamide, represent an important class of S(VI) functionality that is found in pharmaceuticals and agrochemicals (Fig. 1A)4,5,6. Recent medicinal chemistry efforts towards the investigation of bioisosteric replacement groups and the exploration of more sp3-rich chemical space have led to an increased interest in multi-dimensional groups such as sulfonimidoyls (1, 2), the aza-derivatives of sulfonyls7,8,9,10,11,12,13. These S(VI) groups consist of an additional spatial vector and stereogenic sulfur center, have served as bioisoteres for carboxylic acids and sulfonamides7,14, and are known to have favorable physiochemical properties such as permeability and total polar surface area10,11,12,13, making them advantageous for pharmaceutical development.
A Pharmaceutical and catalysis examples. B Synthetic strategies of sulfonimidoyl ureas. C Utilizing t-BuSF as a trifunctional reagent for the asymmetric synthesis of sulfinyl and sulfonimidoyl ureas (this work). TBS = tert-butyldimethylsilyl, PG = protecting group, [M] = metal, 1° = primary, * = chiral center, t-Bu = tert-butyl, i-Pr = isopropyl, e.e. =enantiomeric excess.
One of the first bioactive sulfonimidoyl ureas was reported by Eli Lilly where the observed oncolytic activity was determined to be dependent on the S-chirality of 115. More recently, sulfonimidoyl ureas have taken center stage in the development of NLRP3 inhibitors as immunomodulators16, garnering attention from companies such as Genentech (2)17 and Novartis (DFV-890)18,19,20—with DFV-890 currently under clinical evaluation in multiple USFDA trials21. In addition to promising therapeutics, the S(IV) derivatives have been developed as highly enantioselective organocatalysts for asymmetric aza-Henry reactions (3)22, 1,4-conjugate additions23, and β-amino olefin reductions (4)24,25.
Conventional synthetic approaches for sulfonyl and sulfonimidoyl ureas rely on the addition of the S(VI) functionality to isocyanates at the final stage of a synthesis, prohibiting subsequent derivatization at sulfur (Fig. 1B)15,16,17,18,19,20,26,27. The requisite sulfonimidamides are obtained as racemates through the S(VI) interconversion of N-protected sulfonamides (5) via deoxychlorination and amine addition28, or treatment with organolithium and Grignard reagents to achiral S(IV) electrophiles (e.g., 6, SO2)29,30,31,32 followed by oxidative amination and N-protection. Although additional methods for the synthesis of sulfonimidamides exist33,34,35,36,37,38, they are not commonly employed for sulfonimidoyl ureas. The relatively high step count to key precursors, lack of stereochemical control, and the synthetic restrictions related to isocyanates, make these routes less tractable for future discoveries and manufacturing of this important compound class. Therefore, there is an unmet need to develop practical and modular methods that utilize diverse and readily available building blocks with stereocontrol at sulfur, which was surmised to be possible using a trifunctional chiral S(IV) synthon that can be fully elaborated with carbon and nitrogen nucleophiles (Fig. 1B, right).
Inspired by our chiral bifunctional SuFEx reagent t-BuSF39, we envisioned switching the role of the N,N-diisopropyl urea protecting group through an activation strategy that would introduce a third functional feature of this reagent platform, increasing the overall utility and addressing the current synthetic limitations of sulfonimidoyl ureas (Fig. 1C). Here we disclose an additional reactivity mode of t-BuSF in which the bulky N,N-diisopropylurea serves as an enabling group and undergoes facile sulfinyl urea (7) and sulfonimidoyl urea (8) amine exchange, providing two independent routes for rapid diversification around the S(IV) (9) and S(VI) (10) core with stereochemical control. Moreover, tert-butyl sulfoximines derived from t-BuSF serve as stereogenically stable S(IV) surrogates that circumvent the traditional stereochemical lability associated with sulfinamides40,41 by direct conversion to sulfonimidoyl ureas in a single step. The methods herein have expanded the accessible S(IV) and S(VI) chemical space from t-BuSF, as demonstrated in over seventy examples, have provided significant improvements in the targeted synthesis of clinical candidates and pharmaceutical derivatives, and were applied to a combinatorial chemistry approach for rapid generation of 75 diverse S(IV) and S(VI) derivatives within one workday for a single chemist.
Results and discussion
Reaction discovery
The successful development of t-BuSF as a chiral SuFEx reagent was contingent on an imido protecting group that provided selective S-reactivity as well as reagent and product stability39. By employing a sterically encumbered and electron rich N,N-diisopropyl carbamoyl group, bifunctional asymmetric manipulation at sulfur became possible via sulfinyl urea intermediates, such as 7 (Fig. 2A). The ease in which the protecting group is removed, especially for 2° sulfonimidamides 8 (DMSO/H2O, 60 °C), prompted further investigations into its reactivity and structural features as an additional point of diversification.
A Switching the roles of the t-BuSF protecting group. B Investigating amine exchange efficiency of sulfinyl ureas with N,N-substituents of varying size. aConversion to 9a was determined by LC–MS. bDihedral angle was calculated in Maestro after energy minimizations were performed using MacroModel. C One-pot S-activation and amine exchange of tert-butyl sulfoximines and evaluation of S(IV) sulfinyl urea enantiostability. cEnantiomeric excess (e.e.) was determined by chiral HPLC. dAmine exchange was performed for 3 h at 23 °C. PG = protecting group, EG = enabling group, eq. = equivalents, ND = not detected, TMP = 2,2,6,6,-tetramethylpiperidine, Temp. = temperature.
Conformational analysis of N,N-diisopropyl sulfinyl and sulfonimidoyl ureas revealed out-of-plane distortions about the amide bonds as a result of torsional strain induced by two N-isopropyl substituents (dihedral angle of 159°). In the presence of an α-NH proton, the inherent strain and increased N-pyramidalization42,43,44,45 converts the carbamoyl protecting group (PG) to a strain-activated enabling group (EG) —providing entry to S(IV) (9) and S(VI) (10) derivatives. The torsional strain-release promoted by 1° and 2° amines was found to be highly efficient with N,N-diisopropyl sulfinyl ureas, exhibiting nearly full conversions in three hours at room temperature, while heating (60–80 °C) was required for sulfonimidoyl ureas. The observed thermodynamic barrier for S(VI)-urea amine exchange is presumably due to the tautomeric shift required for N,N-diisopropyl carbamoyl activation along with the decreased amide torsional strain relative to the S(IV) variant.
To investigate this reactivity, a series of phenyl sulfinyl ureas (11) were used to evaluate the effects of torsional strain and steric bulk around the tertiary amide bond (Fig. 2B). Smaller substituents, such as N,N-dimethyl and -diethyl, adopt a near-planar conformation (dihedral angles of 177–175°) and exhibit low reactivity (5–7% conversion to 9a within 3 h) in the presence of morpholine (1 eq.) at room temperature. Conversely, the larger N-isopropyl-N-methyl group’s increased torsional strain (dihedral angle of 172°) revealed slightly higher conversion (10%). The strain-release reactivity trend continued with symmetrical N,N-diisopropyl substituent having the largest change in dihedral angle (159°) and subsequently increasing conversion to 94%. Attempts to exaggerate the torsional strain further with N-tert-butyl-N-isopropyl (dihedral angle of 139°) and 2,2,6,6-tetramethylpiperidine (TMP) (dihedral angle of 105°) proved impractical due to product instability.
Based on our current understanding of the S(IV)-urea amine exchange in conjunction with similar studies of sterically congested electron deficient amides43 and ureas44,45, an addition-elimination pathway at the crowded urea carbonyl is unlikely. Alternatively, elimination of N,N-diisopropyl amine in the presence of a protic amine to form a sulfinyl isocyanate intermediate followed by addition of the less bulky amine is more plausible; although our attempts to identify such an intermediate by analytical or synthetic means have proven unfruitful. Aside from the reaction mechanism, the capriciousness of S(IV) stereocenters under neutral conditions38,39 implored us to examine the effects, if any, this transformation has on the distal chiral center. Enantiopure tert-butyl phenyl sulfoximine 12 was thermally activated using t-BuOK over two hours then neutralized to give sulfinyl urea intermediate 7 that was subsequently treated with morpholine (9a) or aminomethylcyclohexane (9b) at varying temperatures and reaction times (Fig. 2C). To our delight, the stereogenic sulfur center was unaffected at room temperature (21–23 °C) or 60 °C. Although not required, prolonged heating at 80 °C resulted in stereochemical erosion for secondary and tertiary sulfinyl urea derivatives, and eventual decomposition (see Supplementary Information section 1. VII for additional details). The S(IV) stereochemical fidelity analysis suggested enantiopurity would be conserved for reactions conducted at room temperature. The balanced steric influence of N, N-diisopropyl group on S(IV)-urea amine exchange, SuFEx chemistry, and overall chemical stability offers a unique versatile synthetic handle for sulfinyl and sulfonimidoyl synthesis.
Sulfinyl urea scope
With optimal reaction conditions in hand, the scope of the S(IV) reaction was evaluated (Fig. 3). Aryl, heteroaryl and alkyl tert-butyl sulfoximines were prepared from t-BuSF and used as models for scope analysis. Following S-activation of their respective sulfoximines, the resulting sulfinyl urea intermediates were treated at room temperature for three to four hours with amines of varying complexity, from building blocks to advanced pharmaceutical intermediates and drugs. Additionally, the enantiopurity for each class of sulfinyl derivative was determined after isolation.
All reactions were performed on 0.1–0.25 mmol scales unless otherwise stated. Isolated yields are reported. Enantiomeric excess (% e.e.) was determined by chiral HPLC. aTFA (1 eq.) was used as an additive. bCommercial cyclohexyl isocyanate was used. cIsolated as a mixture of diastereomers at the epimeric carbon. Boc = tert-butyloxycarbonyl, Lit. = literature.
Primary aliphatic amines were first evaluated using less sterically hindered benzylic amines and aminomethyl cyclic amines which exchanged smoothly to provide sulfinyl ureas 9b–9e in excellent yields (86–98%), with no observable change in enantiomeric excess (>99% e.e.). Furthermore, amino oxetane 9f and N-Boc protected azetidine 9 g were prepared uneventfully, representing common medicinal chemistry fragments. Interestingly, tert-butyl amine exchanged with N,N-diisopropyl amine in nearly quantitative yield (9h), revealing no steric limitation for primary amine substrates. In addition to primary aliphatic amines, 1-amino piperidine was also a compatible nucleophile giving rise to 9i in 85% yield and providing entry to an underexplored class of sulfinyl ureas.
The decreased basicity and nucleophilicity of aromatic amines contributed to lesser reactivity under optimized conditions, reaching an equilibrium with the starting N,N-diisopropyl sulfinyl ureas and diminished yields (ca. 50–60%). Gratifyingly, Brønsted acid additives were found to enhance the exchange with less basic amines and improving target yields up to 88%. Trifluoroacetic acid (TFA) was selected as the ideal additive due to its role in S-activation and observed reactivity enhancement, making it suitable for one-pot and telescoped protocols. When optimal conditions were employed for aromatic amine substrates (1 eq. of TFA, 2-MeTHF or THF, rt), S(IV) derivatives 9j–9m bearing electron withdrawing/donating groups and ortho-substituents were obtained in 79–86% yields with no erosion of enantiopurity. Additionally, heteroaryl amines, 3-aminopyridine 9n and 2-aminothiazole 9o, were obtained in high yields. Secondary aliphatic amines delivered the target sulfinyl ureas within three hours at room temperature and in high yields without consequence for enantiopurity, as demonstrated by morpholine and N-hexynyl piperazine derivatives 9a, 9p, and 9q. In addition, unsymmetrical secondary amines provide sulfinyl ureas 9r and 9s with functional hydroxyl and alkynyl handles suitable for further downstream manipulation.
Late-stage compatibility and (real-world) functional group tolerance were exemplified using eight pharmaceutically relevant amines of varying complexity. The primary amines of the antiviral oseltamivir and calcium channel blocker amlodipine performed exceptionally well giving sulfinyl urea derivatives 9t and 9u exclusively in the presence of esters, secondary amides and 1,4-dihydropyridine. Additionally, the β-amine of sitagliptin was successfully functionalized to 9v in nearly quantitative yield. An aza-indole sulfinyl urea derivative of a JAK2 inhibitor46 (9w) was made readily accessible by displacement of N,N-diisopropyl amine, introducing S(IV) functionality to commonly encountered kinase pharmacophores. Antibiotic analogs of moxifloxacin (9x) and sarafloxacin (9y) were obtained uneventfully by engaging their 1H-pyrrolo[3,4-b]pyridine and piperazine motifs respectfully. Notable site selectivity was observed for evobrutinib, favoring the piperidine over a diaminopyrimidine leading to the sulfinyl derivative 9z in good yield (76%). Lastly, S(IV)-urea amine exchange was employed as a bimolecular linking strategy between a sulfinyl urea analog of celecoxib and an E3 ligase ligand (9aa) that could be leveraged for the development of proteolysis targeting chimeras (PROTACs).
Synthetic applications of S(IV)-urea amine exchange
Sulfinyl urea organocatalysts impart asymmetric control through chiral hydrogen–bonding environments and have been largely restricted to tert-butyl S-substituents21,22,23,24. These limitations are due in part to the steric bulk offered by the tert-butyl group, availability of the sulfinamide starting material, and most notably, a lack of synthetic methods to efficiently introduce structural and electronic diversity around the chiral S-center. Although t-BuSF could serve as the chiral template for catalyst design, enantiopure (R)-tert-butyl sulfinamide 13 was employed to prepare sulfinyl urea organocatalysts in a single step. Carbamoylation of sulfinamide 13 affords diversifiable intermediate 14 that was directly treated with amines to give sulfinyl ureas 15–17, improving the yield of 16 by 32% and providing a protecting group-free synthesis of 17—subsequently reducing the overall step count22. The practicality and scalability of this method was demonstrated in the gram-scale preparation of 17 and by replacing isocyanates with widely available amine building blocks.
Sulfonimidoyl urea scope
After establishing the sulfinyl urea amine exchange, our focus shifted to the analogous S(VI) transformation of sulfonimidamides. Two secondary sulfonimidamides, N-aryl 8a and N-alkyl 8b, were chosen to examine the reaction scope due to their differences in structure and tautomerization potential (Fig. 4). In general, sulfonimidoyl ureas are less reactive than their S(IV) counterparts and require elevated temperatures to undergo the desired transformation. A similar set of structurally diverse primary and secondary amines were evaluated under thermal conditions (60–80 °C) in THF and MeCN. It was quickly determined that N-aryl derivative 8a undergoes the exchange more readily at 60 °C while N-alkyl derivative 8b required a slightly more elevated temperature (80 °C). Despite the known stereogenic stability of sulfonimidamides47,48, enantiopurity was assessed for each class of sulfonimidoyl ureas.
Both N-substituted N,N-diisopropyl sulfonimidoyl derivatives (8a and 8b) readily reacted with primary aliphatic amines (10a–10h) in good to excellent yields (72–96%) without impacting enantiopurity (>99% e.e.). Benzylic amines and heterocycle-containing primary amines afforded 10a–10c and 10d–10f respectively. Sterically congested valinol provided sulfonimidoyl urea 10g preferentially via S(VI)-urea amine exchange in 84% yield with no observed reactivity at the less hindered alcohol. An arene bioisostere was introduced as a bicyclopentyl (BCP) unit via amine exchange to give S(VI) derivative 10h in high yield. Aromatic amines exhibited similar reactivity trends to the analogous sulfinyl ureas, therefore the same tactic was employed using TFA as an additive. Even though diminished reactivity was observed, good to high yields (56–85%) were achievable for anilinic substrates 10i–10l with no effect on the S-stereocenter. On the other hand, secondary amines readily exchange with N,N-diisopropyl amine producing 10m and 10n uneventfully while maintaining enantiopurity (>99% e.e.). N-Substituted piperazines 10o–10q, 3-hydroxypyrrolidine 10r, and 3-N-Boc-piperidine 10s all delivered the desired sulfonimidoyl ureas in excellent yields. Venturing further away from flat land chemical space, sp3-rich spirocyclic amines were successfully introduced granting access to 10t–10w, which represent untapped chemical scaffolds.
The sulfonimidoyl urea scope was further expanded to encompass the complexity often encountered in drug discovery programs and to verify late-stage introduction of sulfonimidoyl functionality. Amlodipine, alogliptin, and oseltamivir were efficiently exchanged with N,N-diisopropylamine to afford 10x, 10y, and 10z as their respective sulfonimidoyl urea derivatives in the presence of esters, nitriles, amides, and α,β-unsaturated esters. Additionally, the primary aromatic amine of afatinib’s pharmacophore gave rise to 10aa despite the large o-substituent and weak nucleophilicity. Sarafloxacin and moxifloxacin exhibited exclusive reactivity at the secondary amine over condensation with carboxylic acids or 1,4-additions, providing both 10ab and 10ac in 91% yield and 10ad in 85% yield. These representative pharmaceutical examples highlight the selectivity and utility of the sulfonimidoyl urea amine exchange, revealing opportunities for late-stage diversification and derivatization of clinically optimized scaffolds.
While secondary sulfonimidoyl ureas can be functionalized under mild conditions, tertiary substrates were unreactive at elevated temperatures (120 °C, dioxane) or with the addition of Brønsted and Lewis acids (see Supplementary Information section 1. XIII for additional details). To capture this class of sulfonimidoyl derivatives, S(IV)-urea amine exchange and S-activation of sulfinyl ureas were leveraged (Fig. 5). A practical one-pot procedure was developed as a streamlined approach that mitigates the handling of reactive and less stable intermediates while showcasing the degree of modularity and diversity accessible. Tertiary sulfonimidamide 18 was prepared in enantiopure form from the corresponding tert-butyl sulfoximine in a single step (56% yield) via the sulfonimidoyl chloride intermediate. Alternatively, sulfinyl urea intermediates (9) can be fluorinated to provide isolable bifunctionalized S(VI) electrophiles, as demonstrated by sulfonimidoyl fluoride 19, that can undergo additional functionalization. A minor decrease in enantiopurity ( >99% to 99% e.e.) was observed during the fluorination event for 19, however, the stereospecific addition of an amine and turbo-Grignard reagent afforded 20 and 21 in 87% and 76% yield, respectively.
Enantiomeric excess (e.e.) was determined by chiral HPLC. aIsolated yield from tert-butyl sulfoximine via sulfonimidoyl chloride. bIsolated yield from S-activation/flourination of a tert-butyl sulfoximine. cIsolated yield from sulfonimidoyl fluoride 19 using a turbo-amide or turbo-Grignard. dIsolated yield from t-BuSF SuFEx. eYields for each step were not reported.
Synthetic applications
The practical utility and versatility of sulfinyl and sulfonimidoyl urea amine exchanges were further demonstrated in target- and diversity-oriented syntheses of clinically relevant sulfonimidoyl ureas. In two steps, t-BuSF was trifunctionally elaborated in an asymmetric fashion (Fig. 5). An aza-analog of the sulfonylurea antidiabetic drug chloropropamide was prepared in 54% yield from t-BuSF via the trifunctionalization strategy leveraging sulfoximine 22 and n-propyl amine (23). The corresponding S(IV) intermediate 24 was subjected to oxidative amination with t-BuOCl and ammonia providing enantiopure 25 in 63% yield from sulfoximine 22 in a single step. To our knowledge, S-chlorination of secondary sulfinyl ureas has not been reported. The same tactic was applied in a targeted asymmetric synthesis of sulfonimidoyl stereoisomer 1 reported by Eli Lilly15. Starting from t-BuSF, enantiopure sulfoximine 26 was prepared in 74% yield after recrystallization, which was subsequently transformed to 1 in one step (75% yield, > 99% e.e.)—providing an asymmetric route with a decreased step count and improved overall yield. Furthermore, an NLRP3 inhibitor developed by Genentech16 was prepared asymmetrically from sulfoximine 29 that was obtained from t-BuSF in 98% e.e. without recrystallization. Upon S-activation of sulfoximine 29 with TFA, sulfinyl urea 31 was directly exchanged with aniline 30 followed by oxidative amination. This one-step protocol gave rise to sulfonimidoyl urea 32 in 65% yield (45% from t-BuSF) and excellent enantiopurity (98% e.e.), significantly improving the reported 10 step racemic synthesis17 while affording a derivatization platform for future analog development.
One of the most clinically significant sulfonimidoyl compounds to date is Novartis’ NLRP3 inhibitor DFV-890, which is currently under evaluation in six USFDA clinical trials across multiple indications including cancer, heart disease, osteoarthritis, auto-inflammatory syndromes, and COVID-1921. The reported routes to DFV-890 rely on preparative chiral HPLC, isocyanate 33, and sulfonyl to sulfonimidoyl (34) interconversion (Fig. 6A). The requisite sulfonamide 35 is prepared from three different thiazole building blocks (36–38) and sulfur dioxide in varying yields and step counts. Depending on the initial heterocycle chosen, DFV-890 can be prepared in 6–9 steps using at least one protecting group with an overall yield of 0.5–4% and 97.5% e.e. after chiral chromatography.
To address the synthetic drawbacks associated with DFV-890, the trifunctionalization of t-BuSF was applied. Starting from enantiopure (S)-t-BuSF, SuFEx using lithiated thiazole 39 (1st point of diversification) provided tert-butyl sulfoximine 37 with >99% e.e. in 66% yield after recrystallization on gram-scale (Fig. 6B). It is worth noting that the sulfonimidoyl transfer reaction deviated from previously reported conditions39 and was conducted in 2-MeTHF with TMEDA as an additive to aid in dianion formation. Sulfoximine 40 was then subjected to TFA mediated S-activation followed by treatment with an aromatic amine (2nd point of diversification), then oxidative amination with propargyl amine or ammonia (3rd point of diversification) to deliver clickable chemical probe derivatives of DFV-890 41 and 42 in good yields as single stereoisomers, illustrating an advantageous medicinal chemistry route. The same strategy was used in a target-oriented, mock process approach for the gram-scale synthesis of DFV-890 (Fig. 6C). Two different thiazoles (37, 38) were used to prepare gram quantities of enantiopure sulfoximine 40 with no chromatography. S-Activation afforded sulfinyl urea intermediate 43 which was directly converted to DFV-890 via S(IV)-urea amine exchange with aniline 30 followed by in situ oxidative amination providing over one gram of DFV-890 ( >99% e.e.) in 77% yield without the need for chromatographic purification. This modular two-step asymmetric synthesis of DFV-890 reduced the overall synthetic step count (8to 2 steps), improved the yield 10-fold, provided the target compound in enantiopure form, and removed the need for isocyanates, protecting groups, and chromatography.
Combinatorial trifunctionalization of t-BuSF
The modularity, efficiency, and chemical space accessible from t-BuSF were further demonstrated by applying the methods herein to a combinatorial chemistry workflow as a diversity-oriented synthesis platform for S(IV) and S(VI) library generation (Fig. 7A). tert-Butyl sulfoximines (from t-BuSF) serve as the central building block (A) to which the remaining diversity is introduced during sulfinyl urea amine exchange (B) and subsequent S-activation/additions with amines (C) or turbo-Grignards (D). For this compound set, the S-substituent was fixed to phenyl and representatives for building blocks B (maroon), C and D (teal) are shown in Fig. 7A. Individual reactions were set to specific time points (eight total hours, representing a typical workday for a single chemist) and were not independently optimized. Analysis was performed by LC–MS to monitor conversions for yield estimations of target compounds, which were cross-checked by isolation.
A Combinatorial approach to sulfonimidoyl ureas from t-BuSF. Representative nucleophile building blocks were used and reaction profile analysis was performed by LC–MS. B Structural representatives for the sulfonimidoyl classes obtained from each quadrant. LC–MS analysis legend: Dark green =75–100% yield, 15–60 mg of product; light green = 50–75% yield, 10–45 mg of product; yellow = 25–50% yield, 5–30 mg of product; red = 0–25% yield, 0–15 mg of product.
Starting from tert-butyl sulfoximine A, S-activation was carried out on a gram-scale using t-BuOK in 2-MeTHF at 80 °C for 2 h then partitioned into four quadrants (Q1–Q4) throughout a 48-well reaction block. The S(IV)-urea amine exchange was initiated by the addition of 22 amine building blocks (B). After three hours, the newly formed sulfinyl ureas could be split, purified, or further transformed via S-activation; in this proof-of-concept experiment, 44 unique S(VI) products were targeted. Quadrant 1 was subjected to oxidative amination with t-BuOCl and NH3 to obtain 10 primary sulfonimidamides, while quadrant 2 was designed to give 12 products captured in the sulfonimidoyl urea scope (Fig. 4) as controls for method validation. Quadrant 3 contained a diverse set of 12 secondary and tertiary sulfonimidamides, and quadrant 4 was devoted to the synthesis of sulfoximine derivatives via sulfonimidoyl fluorides and turbo-Grignards D1 and D2.
Estimated yields (with the overall mass of target compounds) after four transformations are color-coded within the 48 well plate according to the legend in Fig. 7A. Two examples from each quadrant were isolated and shown to be in agreement with the estimated yields (Fig. 7B). To our delight, 42 examples out of 44 were estimated to have >50% yield (from building block A). The two examples that fell short of the 50% cut off were Q4 sulfoximines treated with D1 having estimated yields of 40%. Within the course of eight hours, 34 sulfonimidamides and 10 sulfoximines were prepared, including 25 sulfinyl ureas, 34 sulfonimidoyl chlorides, and 5 sulfonimidoyl fluoride intermediates. The chemical and structural diversity created within this proof-of-concept workflow signifies the impact t-BuSF will have in the chemical sciences. With the translation to automated liquid handling coupled with the wide variety of available amine building blocks, it is anticipated that high-throughput variations of this approach will be feasible.
In all, a new functional feature of t-BuSF has been developed resulting in a trifunctional chiral SuFEx reagent platform. The key N,N-diisopropyl urea protecting group of t-BuSF was transformed to an enabling group that induces torsional strain-release with primary and secondary amines for the asymmetric synthesis of structurally diverse sulfinyl and sulfonimidoyl ureas. This reactivity mode allows for selective and efficient carbamoyl derivatization with amines at either the S(IV) or S(VI) stage, providing multiple synthetic route options that negates the need for isocyanates and laborious functional group interconversions. The reaction compatibility was explored using myriad amines ranging in structural complexity offering over seventy sulfinamide, sulfonimidamide, and sulfoximine examples with enantiopurities up to >99% e.e. Scalable one-pot protocols were established for rapid construction of the target sulfur functionality in two steps (five transformations) from t-BuSF that were highlighted in five synthetic applications and successfully applied to a combinatorial chemistry workflow. Most notably, the significant synthetic improvements these methods provide for important clinical candidates and derivatives illustrate the impact that the t-BuSF SuFEx platform will have on the discovery sciences. Additional reactivity modes and activation strategies of t-BuSF and intermediates are currently under investigation and will be reported in due course.
Methods
General procedure 1 (GP-1): synthesis of tert-butyl sulfoximines from t-BuSF
To a 50 mL flame dried round-bottom flask equipped with a magnetic stir bar under argon (balloon) was added aryl bromide (1–1.5 eq.) followed by anhydrous Et2O (to make a 0.1 M solution). The mixture was then cooled to –78 °C and n-BuLi (1 eq., 2.5 M in hexanes) or t-BuLi (1 eq., 1.7 M in pentane) was added dropwise and stirred for 1–4 h (for lithium-halogen exchange). t-BuSF (1 eq.) in Et2O was added dropwise at –78 °C and the reaction mixture was stirred at –78 °C for 1–3 h. Upon completion (checked by TLC and LC–MS) the reaction mixture was quenched with MeOH and saturated aqueous NH4Cl solution. The mixture was extracted with EtOAc (3 times) using a separatory funnel. The combined organic layer was washed with water (2 times) and brine (1 time), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude compound was subjected to chromatographic purification to obtain the desired tert-butyl sulfoximines.
General procedure 2 (GP-2): one pot synthesis of sulfinyl ureas from tert-butyl sulfoximines via base-mediated S-activation/amine exchange
tert-Butyl sulfoximines (1 eq.) were taken in a flame-dried scintillation vial equipped with a stir bar and septum. Dry THF (or 2-MeTHF) was added (to make a 0.3 M solution) followed by addition of t-BuOK (3 eq.) at room temperature and the vial was sealed with a cap and Teflon tape. The mixture was heated at 80 °C for 2 h. The reaction mixture was cooled to –78 °C and a solution of TFA (3 eq.) in 2 mL THF (or 2-MeTHF) was added dropwise to make the final reaction concentration 0.1 M. The desired amine (1 eq.) was added to the reaction mixture at room temperature and stirred for 3 h. After completion (monitored by TLC and LC–MS) the solvent was evaporated below 25 °C using a rotary evaporator. The crude compounds were then triturated with Et2O/hexanes two times and decanted or purified via flash chromatography (silica gel) to obtain pure sulfinamides 9.
General procedure (GP-3): One pot synthesis of sulfinyl ureas from tert-butyl sulfoximines via acid-mediated S-activation followed by amine exchange
To a 2-dram vial containing tert-butyl sulfoximine (1 eq.) equipped with a stir bar, a 40% TFA (5 eq.) solution in DCM was added at room temperature. The reaction mixture was stirred at room temperature for 15 min. After completion, monitored by TLC and LC–MS, 2-MeTHF was added to the reaction mixture (to achieve a 0.1 M concentration) and cooled to –78 °C. t-BuOK (3 eq.) was then added portion-wise, and the reaction mixture warmed to room temperature. Amine (1 eq.) was then added at room temperature and the reaction mixture stirred for 3 h. After completion, monitored by TLC and LC–MS, the solvent was evaporated while maintaining a temperature below 25 °C. The crude compounds were then triturated with Et2O/hexanes two times and decanted or purified via flash chromatography (silica gel) to obtain pure sulfinamides 9.
General procedure (GP-4) for synthesis of tert-butyl sulfinyl ureas
Commercially available tert-butyl sulfinamide 13 (1 eq.) was taken up in dry THF (to make a 0.1 M solution) and cooled to 0 °C. NaH (2.5 eq.) was added portion-wise over 5 min. The mixture was stirred at 0 °C for 10 min and carbamoyl chloride, ClCON(i-Pr)2 (1 eq.), was added and stirred at 0 °C for 1 h. After completion (monitored by TLC), AcOH (1.5 eq.) was added to reaction mixture at –30 °C (neutralization) then warmed to room temperature. The desired amine (1 eq.) was then added and stirred for 3 h. After completion (monitored by TLC and LC–MS), the solvent was evaporated and the crude compound purified by silica gel column chromatography to afford the tert-butyl sulfinyl urea.
General procedure 5 (GP-5): Sulfonimidamide amine exchange
Sulfonimidamide 8a or 8b (1 eq.) was taken up in dry MeCN or THF (1.0 mL) in a screw-capped vial followed by the addition of an amine (1 eq.). The reaction vial was securely capped and sealed with Teflon tape then heated to 60 °C (N-aryl substrates) or 80 °C (N-alkyl substrates) for 6–24 h. After completion (monitored by TLC and LC–MS), the solvent was evaporated and the crude compound purified by flash chromatography (silica gel) to afford the desired sulfonimidamide compound 10.
Data availability
All data, including experimental procedures, compound characterization data, and stability analysis data, are available within the article and its Supplementary Information file. Any additional data are available from the corresponding author upon request.
References
Ingenbleek, Y. & Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 71, 413–432 (2013).
Hill, C. R. et al. Sulfur compounds: from plants to humans and their role in chronic disease prevention. Crit. Rev. Food Sci. Nutr. 63, 8616–8638 (2023).
Feng, M., Tang, B., Liang, H. S. & Jiang, X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med Chem. 16, 1200–1216 (2016).
Luzi, L. & Pozza, G. Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol. 34, 239–244 (1997).
Sola, D. et al. Sulfonylureas and their use in clinical practice. Arch. Med Sci. 11, 840–848 (2015).
Liu, Y.-F. et al. A Review on Recent Innovations of Pretreatment and Analysis Methods for Sulfonylurea Herbicides, Crit. Rev. Anal. Chem. https://doi.org/10.1080/10408347.2022.2116694.
Sehgelmeble, F. et al. Sulfonimidamides as sulfonamides bioisosteres: rational evaluation through synthetic, in vitro, and in vivo studies with γ-secretase inhibitors. ChemMedChem 7, 396–399 (2012).
Lücking, U. Sulfoximines: a neglected opportunity in medicinal chemistry. Angew. Chem. Int Ed. 52, 9399–9408 (2013).
Chinthakindi, P. K. et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew. Chem. Int Ed. 56, 4100–4109 (2017).
Frings, M., Bolm, C., Blum, A. & Gnamm, C. Sulfoximines from a medicinal chemist’s perspective: physicochemical and in vitro parameters relevant for drug discovery. Eur. J. Med Chem. 126, 225–245 (2017).
Lücking, U. Neglected sulfur(vi) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front 6, 1319–1324 (2019).
Mäder, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? current status and perspective on an emerging functional group in medicinal chemistry. J. Med Chem. 63, 14243–14275 (2020).
Izzo, F. et al. Exploration of novel chemical space: synthesis and in vitro evaluation of N-functionalized tertiary sulfonimidamides. Chem. Eur. J. 24, 9295–9304 (2018).
Borhade, S. R. et al. Preclinical characterization of acyl sulfonimidamides: potential carboxylic acid bioisosteres with tunable properties. ChemMedChem 10, 455–460 (2015).
Toth, J. E. et al. Sulfonimidamide analogs of oncolytic sulfonylureas. J. Med Chem. 40, 1018–1025 (1997).
Agarwal, S. et al. Discovery of N-cyano-sulfoximineurea derivatives as potent and orally bioavailable nlrp3 inflammasome inhibitors. ACS Med Chem. Lett. 11, 414–418 (2020).
Lai, K. W., Nilewski, C., Pastor, R. M., Stivala, C. Preparation of sulfonimidamide compounds and uses thereof. Patent WO 2023/004257 (2023).
Franchi, L. et al. Preparation of sulfonylurea compound and compositions for treating conditions associated with NLRP activity. Patent WO 2020/102576 (2020).
Coleman, L., Farady, C., Gatlik, E., Schieker, M. Dosing regimen for an NLRP3 inhibitor in the treatment of osteoarthritis. Patent WO 2023/002399 (2023).
Farady, C. J., Gatlik, E., Waldron-Lynch, F. D. Dosing regimen for a NLRP3 inhibitor for treatment of auto-inflammatory syndrome. Patent WO 2024/023696 (2024).
Clinical trials using NLRP3 Inhibitor DFV-890 (National Institute of Health, https://clinicaltrials.gov/study/NCT04868968?term=DFV890&rank=1, https://clinicaltrials.gov/study/NCT06031844?term=DFV890&rank=2, https://clinicaltrials.gov/study/NCT04886258?term=DFV890&rank=3, https://clinicaltrials.gov/study/NCT06097663?term=DFV890&rank=4, https://clinicaltrials.gov/study/NCT05552469?term=DFV890&rank=5, https://clinicaltrials.gov/study/NCT04382053?term=DFV890&rank=6, (2024).
Robak, M. T., Trincado, M. & Ellman, J. A. Enantioselective Aza-Henry reaction with an N-sulfinyl urea organocatalyst. J. Am. Chem. Soc. 129, 15110–15111 (2007).
Kimmel, K. L., Robak, M. T. & Ellman, J. A. Enantioselective addition of thioacetic acid to nitroalkenes via N-sulfinyl urea organocatalysis. J. Am. Chem. Soc. 131, 8754–8755 (2009).
Liu, X. W., Yan, Y., Wang, Y. Q., Wang, C. & Sun, J. Highly enantioselective reduction of β-amino nitroolefins with a simple N-sulfinyl urea as bifunctional catalyst. Chem. Eur. J. 18, 9204–9207 (2012).
Miller, D. Thom, S. St-Gallay, S. Shannon, J. Leeson, P. Preparation of to sulfoximine ureas and sulfoximine thioureas comprising a cyclic group substituted at the α-position useful inhibiting NLRP3. Patent WO 2019/068772 (2019).
Otocka, S., Kwiatkowska, M., Madalińska, L. & Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis. Chem. Rev. 117, 4147–4181 (2017).
De Ventura, T. & Zanirato, V. Recent advances in the synthesis of sulfonylureas. Eur. J. Org. Chem. 2021, 1201–1214 (2021).
Chen, Y. & Gibson, J. A convenient synthetic route to sulfonimidamides from sulfonamides. RSC Adv. 5, 4171–4174 (2015).
Davies, T. Q., Hall, A. & Willis, M. C. One-pot, three-component sulfonimidamide synthesis exploiting the sulfinylamine reagent N-sulfinyltritylamine, TrNSO. Angew. Chem. Int Ed. 56, 14937–14941 (2017).
Davies, T. Q. et al. Harnessing sulfinyl nitrenes: a unified one-pot synthesis of sulfoximines and sulfonimidamides. J. Am. Chem. Soc. 142, 15445–15453 (2020).
Lo, P. K. T. & Willis, M. C. Nickel(II)-catalyzed addition of aryl and heteroaryl boroxines to the sulfinylamine reagent trnso: the catalytic synthesis of sulfinamides, sulfonimidamides, and primary sulfonamides. J. Am. Chem. Soc. 143, 15576–15581 (2021).
Ding, M., Zhang, Z.-X., Davies, T. Q. & Willis, M. C. A silyl sulfinylamine reagent enables the modular synthesis of sulfonimidamides via primary sulfinamides. Org. Lett. 24, 1711–1715 (2022).
García Mancheño, O. & Bolm, C. Synthesis of sulfonimidamides from sulfinamides by oxidation with N-chlorosuccinimide. Beilstein J. Org. Chem. 3, 25 (2007).
Gao, B., Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int Ed. 57, 1939–1943 (2018).
Yu, H., Li, Z. & Bolm, C. Copper-catalyzed transsulfinamidation of sulfinamides as a key step in the preparation of sulfonamides and sulfonimidamides. Angew. Chem. Int Ed. 57, 15602–15605 (2018).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Chem. Eur. J. 26, 12533–12538 (2020).
Luisi, R. & Bull, J. A. Synthesis of sulfoximines and sulfonimidamides using hypervalent iodine mediated NH transfer. Molecules 28, 1120 (2023).
Nandi, G. C. & Arvidsson, P. I. Sulfonimidamides: synthesis and applications in preparative organic chemistry. Adv. Synth. Catal. 360, 2976–3001 (2018).
Teng, S., Shultz, Z. P., Shan, C., Wojtas, L. & Lopchuk, J. M. Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent. Nat. Chem. 16, 183–192 (2024).
Booms, R. E. & Cram, D. J. Stereochemistry of sulfur compounds. III. radical-chain mechanism for racemization of sulfinamides. J. Am. Chem. Soc. 94, 5438–5446 (1972).
Wojaczyńska, E. & Wojaczyński, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Winkler, F. K. & Dunitz, J. D. The non-planar amide group. J. Mol. Biol. 59, 169–182 (1971).
Hutchby, M. et al. Switching pathways: room-temperature neutral solvolysis and substitution of amides. Angew. Chem. Int Ed. 51, 548–551 (2012).
Hutchby, M. et al. Hindered ureas as masked isocyanates: facile carbamoylation of nucleophiles under neutral conditions. Angew. Chem. Int Ed. 48, 8721–8724 (2009).
Ying, H. & Cheng, J. Hydrolyzable polyureas bearing hindered urea bonds. J. Am. Chem. Soc. 136, 16974–16977 (2014).
Chen, L. Preparation of the 2, 4-disubstituted pyrimidine derivative and their medical applications. Patent WO 2020/259683 (2020).
Zhao, P. & Zeng, Q. Progress in the enantioselective synthesis of sulfur (VI) compounds. Chem. Eur. J. 29, e202302059 (2023).
Zhang, X., Wang, F. & Tan, C.-H. Asymmetric synthesis of S(IV) and S(VI) stereogenic centers. JACS Au 3, 700–714 (2023).
Acknowledgements
We gratefully acknowledge the National Institutes of Health (NIGMS R35-GM142577, J.M.L.) for support of this research. This work has also been supported in part by the Chemical Biology Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292, Moffitt). We thank Harshani Lawrence (Moffitt) for NMR and HRMS support, and Alessio Gabellini for the synthesis of several amine starting materials.
Author information
Authors and Affiliations
Contributions
P.A., Z.P.S., and J.M.L. conceived and designed the project. P.A., Z.P.S., A.S., and Y.H. performed the experimental studies. P.A., Z.P.S., and J.M.L. analyzed and interpreted experimental data. Z.P.S., P.A., and J.M.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application naming J.M.L, Z.P.S., and P.A. as inventors has been filed by H. Lee Moffitt Cancer Center & Research Institute, which covers the synthetic methods and development regarding the trifunctionalization of an S(VI) reagent for the asymmetric synthesis of sulfur-containing functional groups. All authors declare no other competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Athawale, P.R., Shultz, Z.P., Saputo, A. et al. Strain-release driven reactivity of a chiral SuFEx reagent provides stereocontrolled access to sulfinamides, sulfonimidamides, and sulfoximines. Nat Commun 15, 7001 (2024). https://doi.org/10.1038/s41467-024-51224-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51224-w
This article is cited by
-
Diversity oriented clicking for modular synthesis
Nature Reviews Methods Primers (2025)