Abstract
Electrocatalytic CO2 reduction to multi-carbon products is a promising approach for achieving carbon-neutral economies. However, the energy efficiency of these processes remains low, particularly at high current densities. Herein, we demonstrate that the low energy efficiencies are, in part, sometimes significantly, attributed to the high concentration overpotential resulting from the instability (i.e., flooding) of catalyst-layer during electrolysis. To tackle this challenge, we develop copper/gallium bimetallic catalysts with reduced activation energies for the formation of multi-carbon products. Consequently, the reduced activation overpotential allows us to achieve practical-relevant current densities for CO2 reduction at low cathodic potentials, ensuring good stability of the catalyst-layer and thereby minimizing the undesired concentration overpotential. The optimized bimetallic catalyst achieves over 50% cathodic energy efficiency for multi-carbon production at a high current density of over \(1.0 \, {{\rm{A}}} \, {{\rm{cm}}}^{-2}\). Furthermore, we achieve current densities exceeding \(2.0 \, {{\rm{A}}} \, {{\rm{cm}}}^{-2}\) in a zero-gap membrane-electrode-assembly reactor, with a full-cell energy efficiency surpassing 30%.
Similar content being viewed by others
Introduction
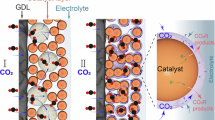
Electrochemical reduction of carbon dioxide/monoxide (CO2/COR) to valuable fuels and chemicals, powered by renewable electrical energy, emerges as a promising approach for achieving carbon neutrality and advancing sustainability transition1,2,3. Currently, achieving practical relevant CO2R/COR current density (>0.2 A cm−2) and selectivity (in regard of Faradaic Efficiency, FE > 80%) for the production of multi-carbon (C2+) products e.g., ethylene (C2H4)4,5, ethanol (C2H5OH)6,7, acetate8,9, and n-propanol10,11, are feasible by employing Cu-based catalysts. However, a noteworthy challenge that often gets overlooked is achieving sufficiently high energy efficiency (EE), especially at high current densities (>1 A cm−2)12,13. To enhance energy efficiency, it’s essential to understand and be able to tune the components of overpotential during catalysis. Generally, the overall cathodic overpotentials (ηtot) primarily consist of three distinct elements: activation (ηact), concentration (ηcon.), and ohmic overpotentials (ηohmic)14. Taking C2H4 production as an example (Fig. 1a), the total overpotential for C2H4 production can be described by Eq. (1):
a illustrations of activation, concentration, and ohimc overpotentials for C2H4 production. b, c The partial current density of C2+, CO, and HCOOH as function of applied potentials. The different colors represented different commercial GDL types. The data were adopted form these literatures: Gd-Cu20, M-H Cu27, Stable Cu23, CuZn13, M-Cu1/Cu NP28, T-Cu29, NiSA/PCFM32, Ni-N/PC0.833, NiPc34, CuPb35, Bi/Bi SnOx36,In-NCN37. Relevant source data are provided in the Source Data file.
Tremendous efforts have been devoted to minimizing these three overpotentials15,16. Various catalyst development strategies, such as tuning oxidation state17,18, alloying19,20, and non-metal element doping21, have been developed for achieving improved activity, resulting in reduced activation overpotential. Besides, tuning microenvironment at the reaction interface has been demonstrated to effectively alter both the activation overpotential and concentration overpotential22,23. Furthermore, there has been extensive recent exploration of the zero-gap membrane electrode assembly (MEA), formed by directly pressing the cathode gas diffusion electrode (GDE) and anode catalyst on each side of the ion exchange membrane, aiming to minimize the ohmic overpotentials across the reactor24,25,26. Despite significant progress achieved through the above strategies, the energy efficiency of CO2R/COR remains in its infancy, particularly at high current densities (EE below ~10–20% at >1 A cm−2)4,12.
To tackle on this grand challenge, we carefully examined the correlations between the apparent current density and the applied cathodic potential using data obtained from the recently developed state-of-the-art Cu-based catalysts (Supplementary Table 1)13,20,23,27,28,29. Surprisingly, even though from different studies, we observed similar potential dependent-current density trends when plotting these data together, as shown in Fig. 1b. Inspired by previous research30,31, we assumed that this trend can be divided into three distinct areas for analysis: a common primary activation-controlled kinetic region within the potential range of approximately −0.2 to −0.7 V versus the reversible hydrogen electrode (VRHE), followed by a mixed-controlled kinetics region (~−0.75 to −0.9 VRHE), and then a severely mass-transport-controlled kinetic region at high potentials (~−0.9 to −1.2 VRHE) for these documented Cu-based electrocatalysts. We reasoned that the onset of CO2 mass transfer limitation, starting from the mixed-control potential region, could be attributed to two factors: (1) insufficient intrinsic CO2 gas transport capability of the commercial GDE materials (such as YLS-30T, SGL-28BC, and PTFE film, etc.,); (2) reaction interface destruction, such as flooding and salt precipitation at the catalyst layer, resulting in substantially increase in concentration overpotential for CO2R and favouring the competing reaction of hydrogen evolution reaction (HER). To validate the first hypothesis, we conducted a similar analysis on the state-of-the-art CO2R systems for CO and formate production32,33,34,35,36,37. We observed that CO and/or formate could be formed at even higher current density within this mixed-control region using the same GDE materials (Fig. 1c, Supplementary Tables 2, 3). Considering that each CO2 molecules requires fewer electrons to form CO and formate compared to other C2+ products, we believe the surface concentration of CO2 on conventional GDE is sufficient to support higher C2+ current. Thus, the observed CO2 mass transport limitations are likely a result of catalyst layer flooding/carbonate salt precipitation, which we believe is, at least partially, associated with the applied cathodic potential. This observation aligns well with recent studies that indicate a correlation between the applied voltage and the H2O content across the GDE layer14,31,38,39. Therefore, if a catalyst candidate can afford a sufficient CO2R current with a small applied cathodic potential before entering the mixed-control region, we can anticipate significantly enhanced CO2R stability, concurrently, enhanced EE at high current densities.
To validate the above hypothesis, it is essential to develop electrocatalysts with enhanced intrinsic activity for CO2R. Previously, bimetallic materials have demonstrated promise in improving the CO2/COR performance through various mechanisms, including electronic effect8,40, strain effect41,42, and geometric effect8,43,44. For example, alloying Cu with different oxyphilic Lanthanide metals can regulate the adsorption energies of key intermediates (*CO and *CHxO) on Cu, thereby enhancing the production of C2H4 and CH445. Therefore, it could be an effective approach for our purpose. Gallium (Ga) exhibits strong oxygen affinity and reduced electronegativity than Cu46,47,48. Besides, previous studies have shown that *CO binds weakly on Ga49,50, thereby, by incorporating Ga into Cu, we anticipate the ability to tune the CO adsorption behavior on Cu, with the aim of achieving enhanced intrinsic activity. During the preparation of our manuscript, a couple of CuGa bimetallic catalysts were studied elsewhere very recently. Raffaella et al. found that the Ga can stabilizes the valence state of Cu at the open-circuit potential (OCV), and the interaction between Cu and Ga greatly improved the structure stability of nanoparticles under the operating conditions of CO2R51. Meanwhile, Han et al. and Zheng et al. found that Cu-Ga bimetallic catalysts can result improved selectivity towards C2+ products, i.e., FE exceeding 70%52,53. Nevertheless, these studies primarily focused on exploring the changes of C2+ product in CO2R selectivity after doping Cu with Ga, here we focus on detailed explorations on whether Ga doping could enhance the intrinsic activity of Cu towards CO(2)R.
In this work, our objective is to develop a guiding strategy to achieve high EE, i.e., reduce activation overpotential and maintain low concentration overpotential. Specifically, we employed an alloying–dealloying strategy to synthesize Cu-Ga bimetallic catalysts with enhanced activity for CO2/CO conversion. Using this CuGa bimetallic material as a model catalyst, we demonstrated that low activation overpotential is critical for achieving high current densities while maintaining low concentration overpotential, consequently, enhancing EE. We found that minor Ga doping onto the Cu surface can lower the activation energy of CO2 to *COOH and binding energy of *CO on the Cu-Ga site, promoting CO production at reduced overpotentials in the CO2R. The resulting increased local CO concentration, combined with the defect-rich sites originated from the dealloying process, significantly boost the C-C coupling process, thereby achieving improved C2+ current density at low overpotentials. As we anticipated, the low activation overpotential of CuGa allows the GDE to operate within the kinetic-controlled region, minimizing the concentration overpotential induced by catalyst layer flooding. As a result, the CuGa-based GDE in this study achieved high cathodic energy efficiency exceeding 50% for C2+ products at high current density of 1 A cm−2. To further reduce the ohmic overpotential, we assessed the CuGa catalyst in a zero-gap MEA reactor. Likewise, enhanced EE were achieved for both CO2R and COR. Particularly for COR, a high current density of 2.5 A cm−2 was achieved at a low cell voltage of ~3 V. At 1 A cm−2, high EE exceeding 35% was obtained for C2+ production surpassing literature benchmarks. Furthermore, as anticipated, the CuGa based-GDE exhibited a stable CO2R and COR performance. It maintained ~90% C2+ Faraday efficiency (FE) during continuous COR operation for up to 120 h (at 0.3 A cm−2), whereas the counter sample Cu-GDE only lasted less than 20 h under identical conditions. Overall, our study demonstrates that optimizing the adsorption behavior of reaction intermediates could effectively reduce activation overpotential for CO(2)R. This reduced activation overpotential, in turn, helps maintain low concentration overpotential, facilitating the energy-efficient electrosynthesis of C2+ products from CO(2).
Results
Synthesis and characterization of Cu and bimetallic CuGa catalysts
The Cu and CuGa catalysts were synthesized via a one-step reduction procedure (see Method). By varying the feed ratio of Cu2+ and Ga3+, a series of CuGa catalyst-precursors were obtained. For instance, the notation of “Cu5Ga1” indicates a Cu2+ to Ga3+ molar ratio of 5:1 was used for synthesizing the catalyst precursor. We also determined the precise Cu/Ga ratio by inductively coupled plasma mass spectrometry (ICP-MS). The ICP-determined Cu/Ga ratios are typically lower than the feed ratio, which is likely resulted from the insufficient doping of Ga during the rapid nucleation and growth of Cu nanoparticles (Supplementary Fig. 1). Note, we chose the sample of Cu5Ga1 as the model catalyst for the following discussions owning to its high activity, vide infra.
From the results of scanning electron microscopy (SEM), the introduction of Ga slightly increased the surface roughness of Cu nanoparticles (Supplementary Fig. 2), which is further confirmed by the measurement of electrochemical active surface area (Supplementary Fig. 3). Transmission electron microscope (TEM) images suggest that (Supplementary Fig. 4) both Cu and Cu5Ga1 exhibit similar particle size distributions ranging from 20 to 50 nm. Additionally, lattice spacing of 0.18 and 0.21 nm were identified for Cu (200) and Cu (111) in high-resolution TEM (HR-TEM) image of as-prepared Cu sample (Supplementary Fig. 5). Likewise, for the as-prepared Cu5Ga1, obvious crystal planes of Cu2O (110) and (200) with lattice spacing of 0.30 and 0.21 nm, respectively, can be observed on their corresponding HR-TEM images (Fig. 2a). Furthermore, electron energy loss spectroscopy (EELS) mapping (with multiple linear least squares fitting) of the as-prepared Cu5Ga1 confirmed the presence of Cu and Ga and their even distributions across the sample particles (Fig. 2a bottom). We also examined the morphology of these catalysts after CO2R assessments, and observed varying degrees of surface fragmentation in all catalysts. Notably, more pronounced degree of fragmentation and reorganization occurred in the CuGa catalysts, marked by the presence of finely spread small particles (Supplementary Fig. 6). Additionally, post CO2R, Cu predominately exhibits Cu (111) facet with high crystallinity, as evidenced by HR-TEM images (Supplementary Fig. 7). As for Cu5Ga1, a more diverse array of facets, including Cu (200), along with numerous disordered boundaries and defects in addition to the Cu (111) facets, is observable (Fig. 2b). We hypothesize that the introduction of rich boundaries and defects is a result of the Cu-Ga alloying and dealloying process during CO2 reduction. In general, these rich boundaries and defects are typically recognized as highly active sites for CO2R, especially for C2+ production54,55,56. Furthermore, EELS mapping of post-Cu5Ga1 confirmed that the presence of Ga on the catalyst surface (Supplementary Fig. 8).
HR-TEM images and EELS-mapping of the (a) as-prepared and (b) post-CO2R Cu5Ga1 catalyst. The PXRD patterns (c) and Cu LMM Auger XPS spectra (d) of as-prepared Cu and Cu5Ga1 catalysts. e High-resolution Ga 2p XPS of as-prepared Cu5Ga1. f Ex-situ XANES at the Cu and Ga K-edge of as-prepared Cu and Cu5Ga1 catalysts. g FT-EXAF spectra at Cu K-edge of Cu and Cu5Ga1 catalyst. h FT-EXAF at the Ga K-edge of the as-prepared and post-catalysis Cu5Ga1. Cu foil, Cu2O, CuO, and Ga2O3 are shown as reference. Post-catalysis XAS of Cu5Ga1was performed immediately after electrocatalytic COR experiment. Relevant source data are provided in the Source Data file.
The powders X-ray diffraction (PXRD) pattern of the as-prepared Cu (Fig. 2c) exhibits three distinctive peaks at 43.4, 50.4, and 74.2°, attributing to Cu (111), (200), and (220), respectively. In contrast, new peaks at 36.5, 42.3, and 61.3° were observed in the as-prepared Cu5Ga1 sample. These patterns further confirm the presence of significant amount of oxidized Cu on the surface of Cu5Ga1, primarily in the form of Cu2O (PDF#77-0199). Notably, no apparent characteristic XRD peaks were not identified for Ga, neither metallic Ga nor oxidized forms of Ga. We attribute this to both the low quantity of Ga doping, as well as the atomic dispersions of Ga into the Cu2O lattices. Similar phenomenon was observed in other related systems20,35. X-ray photoelectron spectroscopy (XPS) was employed to reveal the electronic state of Cu of both Cu and Cu5Ga1 (Supplementary Fig. 9). As expected, we observed XPS peaks at 952.5 and 932.5 eV corresponding to Cu 2p1/2 and Cu 2p3/2, respectively. The shake-up peaks at 945 and 965 eV correspond to the surface native oxide layer of Cu. We analyzed the Cu LMM Auger spectra to evaluate the Cu valence state of the bulk samples of Cu and Cu5Ga1. As shown in Fig. 2d, no apparent Cu0 peak was observed in either Cu or Cu5Ga1. Besides, the oxidation state of Cu in Cu5Ga1 is predominantly +1, lower than that of the as-prepared Cu catalyst (+2). We tentatively attribute this difference to the higher oxophilicity of Ga, preventing Cu in CuGa from being oxidized to CuO under ambient conditions, consistent with the results observed by Raffaella et al.51. High-resolution Ga 2p and Ga 3d spectra were also collected (Fig. 2e, and Supplementary Fig. 10). Separately, the peak position of Ga 3d overlaps with that of K 3p, a common impurity expected in CO2R systems. Hence, we collected and analyzed Ga 2p spectra for more reliable interpretations of the catalyst composition. As shown in Fig. 2e, the two peaks at 1145 and 1119 eV observed for the as-prepared Cu5Ga1 can be assigned to Ga 2p1/2 and Ga 2p3/2, respectively.
To gather more insights to the valence state and coordination environment of Cu in these two samples, both X-ray adsorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) were monitored. As shown in Fig. 2f, the Cu K-edge features of the as-prepared Cu and Cu5Ga1 are closed to the standard samples of CuO and Cu2O, respectively. Besides, the corresponding Fourier transform of EXAFS spectra (FT-EXAF) indicates a Cu-O bond length close to CuO (~1.49 Å) reference for the as-prepared Cu, and a bond length closer to Cu2O reference (~1.39 Å) for Cu5Ga1 (Fig. 2g), aligning with the above XPS analysis. Additionally, the Ga K-edge signal for Cu5Ga1 catalyst before and post COR revealed that Ga mostly exists in the form of Ga2O3. However, following COR, a new peak at ~2.1 Å appears in FT-EXAF spectra (Fig. 2h), indicating the formation of the bimetallic Cu-Ga composition during electrocatalysis, which we believe will quickly convert to Ga2O3 upon cessation of the reaction. Note that, we chose to conduct the operando experiment under COR due to its generally high data quality, i.e., no carbonate formation. However, as CO2 to CO is not the limiting step, we believe the data obtained is relevant for our analysis.
Determining the intrinsic activity of CuGa catalyst towards CO2R
The as-prepared Cu and Cu5Ga1 catalysts were assessed for CO2R under identical conditions. We first conducted a series of CO2R measurements under different temperatures to explore the intrinsic activities of these two catalysts toward CO2R (H-cell, 0.1 M KHCO3, and −1.0VRHE). Note that temperature can affect CO2 solubility in aqueous electrolyte57, we thus carefully determined the temperature window to ensure that there were no apparent CO2 mass transfer limitations, at least at our test potentials (−1.0 VRHE). As shown in Fig. 3a, b, Cu5Ga1 exhibits a similar product distribution as Cu, including ethylene (C2H4), ethanol (C2H5OH), methane (CH4), formic acid (HCOOH), etc. However, Cu5Ga1 demonstrates enhanced selectivity and partial current density towards C2+ products and suppressed HER compared to the reference Cu catalyst at all tested temperatures. Furthermore, we plotted the trends of the selectivity, measured in terms of Faraday efficiency (FE) of each product as a function of the electrolyte temperature (Supplementary Fig. 11). We found that the FEs of both H2 and C2H4 decrease as the temperature decreases, while the FEs of CH4 and C2H5OH exhibit the opposite trend. These different trends may result from direct and/or indirect effects of temperature, such as changes in CO coverage, local pH, and surface structure, etc.58. As ethylene is the major C2+ product, we first plotted the logarithmic dependence of ECSA normalized partial current density of C2H4 with reciprocal of temperature to estimate the corresponding activation energy (Ea) based on the Arrhenius equation (2). The same analysis was conducted for H2 production as it is the major competing reaction.
where i is the partial current density of the specific product, Ea is the corresponding activation energy, R is the ideal gas constant, T is the reaction temperature, and A is the pre-exponential factor58. Consequently, as depicted in Fig. 3c, Cu5Ga1 exhibits a substantially lower Ea of 28.5 kJ/mol at −1 VRHE towards C2H4 production compared to that of Cu (33.1 kJ/mol at −1 VRHE) under identical conditions. Moreover, Cu5Ga1 also demonstrates much higher Ea for H2 production (39.7 kJ/mol at −1 VRHE) compare to that of Cu (27.3 kJ/mol at −1 VRHE, Fig. 3d). Taken together, one can anticipate that Cu5Ga1 can inhibit the competing HER during CO2R and facilitate the production of C2+ products, particularly C2H4.
CO2R current densities and FEs for all measurable products at different temperatures at applied potential of –1.0 VRHE in 0.1 M KHCO3 for Cu5Ga1 (a) and Cu (b). a, b No iR correction. The corresponding Arrhenius plots for C2H4 (c) and H2 (d) productions during CO2R. The ECSA for Cu and Cu5Ga1 in H-cell is 83.4 and 91.3 cm2, respectively. CO2R product distribution using different CO2 partial pressure at current density of 0.5 A cm–2 for Cu (e) and Cu5Ga1 (f), measured in flow cell using 1 M KOH as the electrolyte. gThe applied potential (left) and the ratio of FEC2+ to FEH2 (right) under different CO2 partial pressure for CO2R on Cu and Cu5Ga1 under different current density. 100% iR correction was applied to calculate the applied potential. The solution resistance (R): Cu-100, 3.23 ± 0.9 Ω; Cu-500, 2.61 ± 0.6 Ω; Cu5Ga1-500, 2.21 ± 0.8 Ω; Cu5Ga1-1000, 2.06 ± 0.4 Ω. Details about the recorded process can be found in the Method section. h The FEC2+ for CO2R on Cu and Cu5Ga1 catalysts at different current densities with different CO2 partial pressure. The error bars in a–d were standard deviations which determined from at least 3 independent experiments. Relevant source data for this figure are provided in the Source Data file.
To further assess the performance of Cu5Ga1 under practical relevant conditions, we conducted CO2R in a flow cell configuration (Supplementary Fig. 12) and employed diluted CO2 streams. As shown in Fig. 3e, f, we present the product distributions of Cu5Ga1 and Cu at the same current density of 0.5 A cm−2, under different CO2 partial pressure using N2 as the inert balance gas. At 100% CO2 partial pressure, Cu5Ga1 (FE = ~68%) exhibits slightly lower C2+ selectivity compared to Cu (FE = ~80%), which can be attribute the smaller overpotential applied. However, upon reducing the CO2 partial pressure from 100% to 75%, the FEC2+ of Cu decreased rapidly (to ~40%), coupled with a significant increase in FECH4 (from ~5% to ~30%). This result indicates that flooding likely occurred at the GDE surface. Notably, the applied potential was recorded to be approximately −0.87 VRHE (Fig. 3g left) for Cu at 75% CO2 partial pressure, aligning with the mass transport region we proposed earlier (Fig. 1b). When we further reduce the CO2 partial pressure to 50% and 25%, H2 became the predominate product for Cu, as anticipated (Fig. 3g right). In contrast, Cu5Ga1 catalyst exhibits a distinct behavior. Specifically, as the CO2 partial pressure decreases, the FEC2+ increases instead of decreasing, reaching the highest FEC2+ (~83%) and FEC2H4(~53%) at 50% CO2, while the FEH2 only increases modestly from 4% to 8%. This intriguing trend further supports our hypothesis on the potential-dependent regions for electrode flooding, as the applied potential for Cu5Ga1 is only −0.7 VRHE at 50% CO2. When we further reduce the CO2 partial pressure to 25%, the applied potential increased significantly to compensate for the CO2 concentration overpotential. Consequently, we observed a significant decrease in FEC2+ (~60%), and increase in selectivity towards to CH4 and H2, indicative of flooding at the GDE reaction layer.
To further validate the hypothesis on the potential-dependent electrode flooding behavior, we varied the applied potential for both Cu and Cu5Ga1 based on our preliminary categorization of different kinetic regions (Fig. 1b). As shown in Fig. 3g, we chose current densities of 0.1 and 0.5 A cm−2 for Cu, and current densities of 0.5 and 1 A cm−2 for Cu5Ga1 for this assessment, respectively. At the current density of 0.1 A cm–2, applied potentials for Cu at different CO2 partial pressure lie in the kinetic and mixed-control region, indicating flooding may not occur significantly. These applied potentials are similar to those applied to Cu5Ga1 at 0.5 A cm–2, as anticipated, we observed similar trends in the ratio of CO2R/HER (Fig. 3g right) and the changes of FEC2+ (Fig. 3h and Supplementary Fig. 13). To drive a higher current density, as depicted in Fig. 3g, the applied potentials for both Cu5Ga1(1 A cm−2) and Cu (0.5 A cm–2) quickly enter the mixed-control region (negative than −0.75 VRHE) and then the severely flooded region as CO2 partial pressure decreases. As expected, we observed a rapid decrease in both the ratio of FECO2R/FEH2 and the overall FEC2+ (Fig. 3g, h). These correlations indicate that the GDE reaction interface stability regarding flooding is most likely related to applied potential. Taken together, our results suggested that the introduction of Ga can significantly reduce the activation overpotential for the production of C2+ products, e.g., C2H4, and inhibit HER process. Benefiting from the reduced activation overpotential, the overall applied cathodic potential is sufficiently low in the case of Cu5Ga1 for achieving high current density for C2+ production while preventing sever electrode flooding.
Exploring the origin of improved activity of the CuGa catalyst
In addition to the morphological changes (Fig. 2d), we also observed that the presence of the Ga exhibits a noticeable effect on preventing Cu oxidation under ambient conditions, as evidenced by XRD, Cu 2p XPS, and Cu LMM Auger results (Supplementary Figs. 14 and 15). We believe this effect is likely due to the high oxophilicity and low electronegativity of Ga. Furthermore, we observed a substantial loss of Ga from the CuGa catalysts after CO2R, as evidenced by EELS fitting, Ga 2p XPS, and ICP-MS (Supplementary Figs. 8, 16, and 17). We propose that the Ga loss can be attributed to two factors. Firstly, it may occur during the alloying–dealloying process. Secondly, the hydrophobicity of the GDE electrode decreases upon reaction cessation, leading to the etching of the Ga2O3 layer by KOH. Nevertheless, we believe the residual minor Ga on the catalyst surface could still play a significant role during catalysis. We will delve into a detailed discussion on this aspect thereinafter.
We also investigated the dynamic changes in Cu and CuGa catalysts under working conditions by in-situ XANES. Due to the insufficient signal-to-noise ratio caused by interfacial instability in alkaline CO2R reaction (i.e., carbonate formation) under practical relevant current densities, we opted to perform COR instead of CO2R, since the CO2 to CO step is most likely not the limiting step on both Cu and CuGa in our experimental conditions59. As shown in Fig. 4a, the Cu K-edge normalized absorption spectra of Cu5Ga1 and Cu catalysts at the OCV state reveal that in the as prepared Cu5Ga1 sample, Cu exists predominately in the state of Cu2O, whereas in the as synthesized Cu catalyst, it mostly exists in the state of CuO (Supplementary Fig. 18). Upon the application of a negative potential to initiate the COR, the Cu matrix of both catalysts undergoes a swift conversion from oxidized states to the metallic state during electrocatalysis. While previous reports suggest that the presence of Cu+1 can promote C2H4 selectivity and stability60,61, there is insufficient evidence to support the notion that Ga can help maintain the +1-oxidation state in our system. As depicted in Fig. 4b, FT-EXAF conversion reveals that at applied negative potentials (–0.48, and –0.63 VRHE), the length of Cu-Cu bond in Cu5Ga1 (~2.25 Å) slightly exceeds that of the standard Cu foil (~2.18 Å). As the potential becomes more negative, the Cu-Cu bond length returns to the standard length. However, obtaining high-quality in-situ signals for the Ga K-edge was not successful due to its low content and significant background fluorescence interference from Cu35,62. Nonetheless, Cu-Ga bond can be clearly observed in the same measurement after COR reduction (Fig. 2h). Taking into account the theoretical potentials for Ga3+/Ga0 (EGa2O3/Ga0 = –0.485 VRHE, EGaOOH/Ga0 = –0.493 VRHE, EGa(OH)3/Ga0 = –0.415 VRHE)63 and the above results, we reasonably speculate that the above potential-dependent change in Cu-Cu bond length is a result of alloying and dealloying processes. We believe that Ga tends to redeposit onto the Cu lattice during catalysis, consistent with similar observations made elsewhere51.
a In-situ XANES at the Cu K-edge of Cu5Ga1 catalysts during COR condition (1 M KOH, flow cell) with different applied potentials. 100% iR (4.3 ± 0.3 Ω) correction was applied to calculate the applied potential. b the corresponding FT-EXAF spectra at Cu K-edge of Cu5Ga1 catalyst. Cu foil is shown as reference. In-situ ATR-SEIRAS spectra of Cu (c) and Cu5Ga1 (d) during COR at different applied potentials. 100% iR correction was applied to calculate the applied potential. The solution resistance (R) for Cu and Cu5Ga1 is18.2 and 10.2 Ω, respectively. e Potential-dependent COad peak area on Cu and Cu5Ga1 in in-situ ATR-SEIRAS spectra. e The partial current density of CO (top), C2+ products(middle), and its sum (bottom) in CO2R reduction (1 M KOH, flow cell) under different applied potential. 100% iR correction was applied to calculate the applied potential. The solution resistance (R) for above analysis can be found in figure caption of supplementary Fig. S22. Details about the recorded process can be found in the Method section. f The free energy surface (FES) for CO2 reduction to CO and CO-CO coupling with Cu (100) surface with 0.0, 1/6, and 1/4 ML of Ga. Right sides are the structures of the *OCCO on the1/6 ML Ga and pristine Cu (100). Atom color code: Cu, dark yellow; Ga, light purple; C, black; O, red; H, white. Relevant source data are provided in the Source Data file.
Attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) was employed to monitor adsorption behavior of *CO, a key intermediate for CO2/COR reaction. Previous work has suggested that the CO2 to CO steps is not rate-limiting on Cu for C2+ products59. Therefore, to eliminate the influence from the CO2 to CO steps, we carried out IR measurements directly under the COR conditions to monitor the *CO on Cu5Ga1 and Cu, ensuring a fair comparison. As shown in Supplementary Fig. 19a, b, as the cathodic potential increases from OCV, a distinct band in the range of 2000 -2100 cm–1, attributed to the atop-bound CO (COad) on the catalyst surface, emerged on both Cu5Ga1 and Cu. Note, the observed COad peak are well described by a commonly used model consisting of the “low frequency band” and the “high frequency band” (Fig. 4c, d)64,65. Assuming Beer’s law holds, the integrated COad peak area is directly proportional to the COad coverage66. In this case, one could observe that the COad coverage on Cu5Ga1 is higher than that of Cu (Supplementary Fig. 19c). Noted, other factors, such as surface enhancement efficiency, ECSA of catalysts, etc., will also affect this high “COad peak area”. On the other hand, the wavenumber of COad is more closely associated with the COad binding strength on a given catalyst67. As a result, we observed that the wavenumbers of both COad peaks on Cu5Ga1 consistently remain slightly higher than those on Cu (Supplementary Fig. 19d), indicating that the binding strength of COad on Cu5Ga1 is slightly lower than on the pristine Cu.
To further understand the effect of CO adsorptions, we analyzed CO generation during CO2R and found that Cu5Ga1 indeed exhibited substantially higher selectivity and partial current density towards CO, particularly at low potentials compared to Cu (Supplementary Fig. 20). As shown in Fig. 4e, Cu5Ga1 consistently exhibits a higher partial current density (CO and C2+ combined) than that of Cu across a broad overpotential window, even when normalized to its higher ECSA. Given the minimal production of CH4 and HCOOH, we believe this enhanced intrinsic activity of Cu5Ga1 is most likely resulted from its weaker COad binding strength and more favorable COad activation coupling step.
We then conducted density function theoretical (DFT) calculations on CuGa catalyst to further understand the improved intrinsic activity of the bimetallic CuGa catalyst. The corresponding optimized computational model structure of Ga on Cu (100) are provided in Supplementary Data 1. Despite the experimentally determined Ga/Cu ratio in post-Cu5Ga being ~1:20, we found that Ga doping at the topmost layer is energetically favored by 0.43 eV than at the subsurface, which is substantial. Therefore, a higher Ga concentration on the surface of CuGa catalysts should be expected. However, determining the actual coverage of Ga is challenging. Alternatively, we have considered four different Ga coverages on Cu surface: 0.0 ML, 1/6 ML, 1/4 ML, and 1/2 ML. On the pristine Cu (100) surface, we found that CO prefers to be adsorbed on the hollow site, which is more stable by 0.06 eV than the bridge site, and by 0.13 eV than the atop site. The calculated free energy barrier for CO-CO coupling is 0.76 eV, aligning well with the previous calculations68. In a recent work, the Ga-doping on Cu(111) surface was found to promote the CO adsorption52. We found that CO adsorption at the Ga site on Cu (100) is suppressed rather than promoted. The adsorption energy of CO at the Ga site was calculated to be 0.86 eV higher than that of at the Cu atop site on the pristine Cu (100) surface. Additionally, after structural optimization, all the CO molecules initially placed at the Cu-Ga bridge site or the 3Cu-Ga hollow site will diffuse to the Cu atop site. Therefore, the most favorable adsorption site for CO is expected to vary with the Ga coverage. With 1/6 ML Ga, CO can still park at hollow sites that are not adjacent to Ga. However, with 1/4 ML and 1/2 ML Ga, the hollow site becomes coordinated to at least one Ga atom, making the atop site becomes the favorable site for CO adsorption. In all, as the Ga coverage increases, we found a weakening in the adsorption of all the reaction intermediates (Fig. 4f). For instance, the adsorption free energy of CO on the pristine Cu (100) surface is –0.32 eV, and it is weakened to –0.23 eV, –0.04 eV, and 0.12 eV with 1/6 ML, 1/4 ML, and 1/2 ML of Ga, respectively. Besides, Bader charge analysis reveals that each Ga atom doped on Cu (100) surface will donate about 0.22 electrons to the surrounding Cu atoms due to the lower electronegativity of Ga compared to Cu. It has been previously demonstrated that the interaction between CO and Cu is governed by both the π-bonding and σ-repulsion69, with the latter being sensitive to the occupancy of Cu sp-band. Consequently, the electrons donated by Ga occupy more Cu sp-bands, resulting in higher σ-repulsion and, therefore, weaker CO adsorption.
Owning to the weakening of CO adsorption, the free energy surfaces suggest that the surface can efficiently reduce CO2 to CO (Fig. 4f, Supplementary Fig. 21). As a result, we found that the effective barriers for CO-CO coupling to form OCCO are substantially lowered upon Ga-doping. Specifically, the reaction barriers are reduced by 0.08 eV, 0.10 eV, and 0.05 eV with Ga coverages of 1/6 ML, 1/4 ML, and 1/2 ML, respectively, suggesting that Ga-doping can promote the formation of C2+ products across a wide range of Ga coverages. We believe the weakened adsorption of CO leads to reduced activation energy required for reaching the transition state for CO-CO coupling or forming other activated CO states, resulting in enhanced activity towards C2+ products as we observed experimentally.
Energy efficiency assessment of CO2/COR on Cu5Ga1
After pinpointing the enhanced activity of the CuGa catalysts, we proceeded to validate its performance for CO2/COR reaction in more practical relevant reactors. We first analyzed the product distributions for both CO2R and COR on CuGa (Supplementary Figs. 22–27) and compared them to those observed on pristine Cu to investigate the effect of Ga-doping on C2+ products selectivity. As shown in Supplementary Figs. 26, 27, we observed similar products distributions, i.e., ethylene-to-oxygenates ratios, on both Cu5Ga1 and Cu, for both CO2R and COR before flooding occurs. However, as the current density increased, this ratio on Cu5Ga1 only increased slightly, while the ratio on pristine Cu increased dramatically due to the decrease in liquid products. This is likely induced by electrolyte flooding caused by the rapidly increased cathodic potential for the pristine Cu. Separately, favored liquid C2+ products (~50%), especially for acetate (~20%), were observed in COR compared to CO2R (liquid C2+ products ~30%, acetate ~5%) on both CuGa and Cu. We believe this observation is likely attributed to the higher local pH and CO partial pressure during COR (Supplementary Note 1, Supplementary Table 4)70. Taken together, we believe that Ga-doping into Cu predominately facilitates the kinetic rate-limiting step (*CO activation) and consequently enhances the overall C2+ production activity. As this kinetic rate-limiting step precedes the selectivity-determining step for C2+ production, we did not observe an obvious difference in terms of C2+ selectivity and ethylene-to-oxygenate ratios between CuGa and Cu, for both CO2R and COR. Consequently, as shown in Fig. 5a, the optimal CuGa catalyst (Cu5Ga1) achieves high FEC2+ (> 80%) and low FEH2 (<~5%) (Fig. 5b) across a wide current density range of 0.8−1.3 A cm−2 in CO2R. The excellent performance of Cu5Ga1 is even more pronounced in COR, achieving more than 85% FEC2+ selectivity in the wide current density range of 0.5−1.5 A cm−2. Furthermore, we also found that, for both CO2/COR, CuGa catalysts achieve the highest FEC2+ at much lower overpotential compared to Cu. Notably, the decrease in overpotential is more pronounced in the case of COR. For instance, at small current densities (0.1−0.3 A cm−2) where flooding may not occur, or at least not severely, the reduction in overpotentials already exceeds 100 mV. (Fig. 5c, Supplementary Fig. 28).
The FEC2+ (a), FEH2 (b) of Cu and Cu5Ga1 in CO2 (solid) and COR (hollow). c The FE C2+ distribution of two catalysts at different applied potentials in CO2/COR. d Partial current densities of C2+ products of Cu and Cu5Ga1 in CO2 /COR. e The normalized partial current density of C2+ as function of applied potentials for four catalysts in CO2R. f The EE1/2 for two catalysts at different current density under CO2R. a–e The CO2/COR tests were performed in flow cell with 1 M KOH as electrolyte, Ni foam as counter electrode, and the flow rate of electrolyte and CO2/CO feeding is 25 mL/min. g Stability test at −0.3 A cm−2 current density in flow cell for Cu and Cu5Ga1, the anode electrolyte was regularly renewed. a–g 100% iR correction was applied to calculate the applied potential. The solution resistance (R) for above analysis can be found in figure caption of supplementary Figs. S22 and S23. Details about the recorded process can be found in the Method section. h The current density for two catalysts at different cell voltages; purple area for COR with 2 M KOH as anode electrolyte; pink area for CO2R with 1 M KOH as anode electrolyte. h The CO2/COR tests were performed in MEA with different electrolytes, IrOx/Ti mesh as counter electrode, and the flow rate of CO2/CO is 25 mL/min. The error bars in a-f were standard deviations which determined from at least 3 independent experiments. Relevant source data are provided in the Source Data file.
Our attention also extended to the production of H2 and CH4, two representative indictors of interface instability in Cu-based systems71. We found that ~ −0.75 VRHE emerged as a turning point in our measurements for both CO2/COR (Fig. 5d, Supplementary Figs. 29–31). When the applied potential went below ~ −0.75 VRHE, both the FEH2 and FECH4 quickly increased for all catalysts in both CO2/COR, indicating flooding occurred at the reaction interface. Additionally, the ratios of FEC2+ to FEC1+ (C1 includes both CO and CH4) at different potentials also exhibit the same trends (Supplementary Fig. 32). Overall, these observations align well with our hypothesis on potential-dependent electrode-flooding behavior, and the high intrinsic activity of CuGa catalysts ensures substantially enhanced current density can be achieved with sufficiently low cathodic potentials (Fig. 5e, Supplementary Figs. 33, 34). As a result, showing in Fig. 5f, Cu5Ga1 achieves an encouragingly high cathodic EE of >50% towards C2+ products at a high current density of 1 A cm−2, surpassing that of Cu (~30%) significantly. Even at a further increased current of 1.5 A cm−2, Cu5Ga1 maintained over 40% cathodic EE1/2 for C2+ products, while that of Cu rapidly dropped to less than 10%.
Single-pass carbon conversion efficiency (SPCE) serves as another crucial indicator for evaluating the CO2R performance. To mitigate the severe CO2 loss in alkaline electrolyte, we assessed the CO2R performance and SPCE in acidic electrolytes (0.5 M K2SO4, pH = 2). As anticipated, Cu5Ga1 catalyst demonstrated substantially higher C2+ selectivity and lower overpotential at all current densities compared to those observed on Cu, confirming its enhanced performance observed in alkaline CO2R (Supplementary Fig. 35). Furthermore, at the optimal CO2 flow rate, an appreciable SPCE of 40% could be achieved at a current density of 500 mA cm−2 in Cu5Ga1-based CO2R in acidic electrolyte.
Stability is equally crucial for CO(2)R. In the CO2R, Cu GDE rapidly flooded due to the higher cathodic potential required for 0.5 A cm−2, while the Cu5Ga1 GDE could last for significantly longer time under the identical operating current density (Supplementary Fig. 36). Considering that salt crystallization in the CO2R also contributes to destabilize of the GDE reaction interface. Hence, we focused on assessing the impact of electrode potential on the stability of COR (Fig. 5g). The results revealed that the Cu5Ga1-GDE can maintain ~90% FEC2+ at an industrial current density (0.3 A cm−2) for more than 120 h, while the Cu-GDE experiences system failure at ~20 h. Cu-based catalysts may undergo degradations due to dissolution, restructuring, and fermentations, which can lead to unsatisfactory stability for long-term COR electrolysis72,73. Therefore, to explore the origin of the instability of pristine Cu electrode, we carried out detailed analysis on the changes in ECSA, product distributions, and OH− adsorption of the two catalysts during and after COR for various time periods (Supplementary Figs. 37–39). As a result, we conclude that the electrolyte flooding is the predominant cause of the instability of pristine Cu during long-term COR electrolysis, despite a certain level of surface restructuring occurring in both catalysts.
We proceeded to assemble a MEA reactor to minimize the ohmic overpotential and assess the performance of CuGa catalysts for CO2/COR by using different anode electrolytes. In the CO2R reaction, resembled those obtained in the flow-cell, the Cu5Ga1 catalyst demonstrated a low cell voltage towards CO2R products than that of Cu. Specifically, at a current density of 0.8 A cm−2, the cell voltage observed in Cu5Ga1-MEA was ~134 mV and 247 mV lower compared to those of Cu-MEA, in 0.1 M KHCO3 and 1 M KOH, respectively (Fig. 5h, Supplementary Figs. 40–43). In the COR reaction, both Cu and Cu5Ga1 exhibited enhanced activity compared to CO2R and achieved higher FEC2+ (~90%) across a wide current density range of 0.1–1.5 A cm−2 (Supplementary Fig. 44). More remarkably, benefiting from the lower cell voltage, Cu5Ga1 catalyst can achieve a high current density of 2.5 A cm−2 at a cell voltage of 3 V and still maintaining a ~70% FEC2+ (with a partial current density of C2+ of 1.6 A cm−2). As expected, the highest EE (~40%) of Cu5Ga1 in COR can be achieved at 0.8 A cm−2, and it can maintain ~30% at high current density (2 A cm−2). In contrast, Cu-MEA will quickly shift to the HER due to system instability at current densities exceeding 1.5 A cm−2. As for the EE in CO2R, Cu5Ga1 also maintains high full-cell EE at larger current densities, compared to Cu (Supplementary Fig. 45). Additionally, upon comparing the EE1/2 and full-cell EE achieved in the flow cell and MEA with the latest research results, we found that the Cu5Ga1 catalyst in this study demonstrated advantages in energy efficiency especially at high current densities (Supplementary Fig. 46 and Tables 5, 6).
Discussion
In conclusion, we observed that the currently low energy efficiency for CO(2)R is largely resulted from the high concentration overpotentials induced by GDE instability (i.e., flooding issue), particularly when under high cathodic potential is applied to drive high current density. To mitigate this challenge, we developed a bimetallic CuGa catalyst with a reduced activation overpotential for producing C2+ products. This improvement is attributed to the combination of the electronic effect of Ga doping and the resulting undercoordinated Cu sites during catalysis. As a result, the Cu-Ga based GDEs could achieve substantially high CO(2)R current density with sufficiently low overpotentials. This low overpotential, in turn, enables the energy-efficient and stable operation of CO(2)R at high current densities. For instance, using the optimized Cu5Ga1 catalyst, we the achieved over 50% cathodic energy efficiency towards C2+ at a high current density exceeding 1.0 A cm−2. Moreover, when employing this Cu5Ga1 catalyst in a MEA reactor, a notable COR current density of 2 A cm−2 was realized at a modest cell voltage of 3 V, corresponding to a full cell energy efficiency of over 30%. Overall, the correlation between the electrode potential and the stability of the reaction interface, along with the strategy proposed in this study, opens a pathway for the energy-efficient electrosynthesis of C2+ products from CO2/CO.
Methods
Chemicals
Copper (II) chloride dihydrate (CuCl2 ·2H2O, ≥99.0%), sodium borohydride (NaBH4, 99.99%), hydrochloric acid (HCl, GR), Gallium (III) oxide (Ga2O3, 99.99%), Polytetrafluoroethylene solution (PTFE, 60 wt%) and isopropyl alcohol (IPA, 99.5%), NafionTM D520 (5 wt% in EtOH) were purchased from Sigma-Aldrich. All chemicals were used without further purification. Gallium chloride (GaCl3, 99.99%), potassium hydroxide (KOH, 99.99%), potassium bicarbonate (KHCO3, 99.99%) were purchased from Macklin. Anion-exchange membrane (Sustainion X37-50 RT Grade) was purchased from Dioxide Materials. The carbon paper with microporous Layer (YLS-30T), pure carbon paper (TGP-H-060), and IrOx/Ti mesh were purchased from Suzhou Sinero Technology company. All chemicals were used as received. Ultra-pure water (18.2 MΩ. Cm, Millipore) was used throughout the experiments.
Characterizations
Ex-situ characterization
The surface morphology of as-prepared and post reaction catalysts were characterized by field scanning electron microscopy (FE-SEM, SU-8000, Hitachi, Tokyo). Transmission electron microscopy (TEM) image and EELS mapping were obtained by JEOL JEM-2010F (Japan). X-ray diffractometer (XRD, Bruker D8-advance) was applied to characterize the crystal structure with a Cu K-α as X-ray source. X-ray photoelectron spectroscopy (XPS) was conducted to analysis the surface chemical compositions on an Escalab 250 Xi (America) with an Axis Ultra DLD spectrometer (resolution 0.5 eV). In-situ ATR-SEIRAS spectra during COR at different applied potentials (0.5 M KHCO3 as the electrolyte) was measured by Fourier transform infrared spectroscopy (FTIR, IRTracer-100, Shimadzu).
The catalysts were dropped on a gold-coated silicon table as a working electrode. Ex-XAS and in-situ XAS were performed at XAFCA beamline of Singapore Synchrotron Light Source under transmission/fluorescence mode. Cu and Ga K-edge XANES spectra were collected. Athena was used for data process. Inductively coupled plasma optical emission spectroscopy (ICP-OES, Thermo Scientific™ iCAP™ 7400) analysis was applied to quantify the mass of Cu and Ga on the electrodes.
Catalysts synthesis
The Cu and CuGa presuors were synthesized via one-step reduction process. Specifically, the Cu5Ga1 was synthesized as follows. The reduction solution was first prepared, including 378.3 mg NaBH4 and 10 mL H2O. And then prepared the CuGa precursor solution, dissolved 255.0 mg of CuCl2 ·2H2O in 3 mL of dilute hydrochloric acid (2 M), and then add 1 mL of GaCl3 (0.3 M) solution. The resulting mixed CuGa solution was sonicated for 5 min and then added dropwise to the NaBH4 reduction solution in an ice bath. After stirring for 1 h, the black solid is collected by centrifugation, washed with ultrapure water and IPA, and dried in a vacuum oven at 60 °C. To obtain Cu10Ga1 and Cu5Ga2, the add volumes of GaCl3 solution were 0.5 and 2 mL, respectively. Cu was obtained without GaCl3 solution. The other steps were same to the synthesis procedure of Cu5Ga1.
Electrochemical measurements
All electrochemical tests were performed using BioLogic VMP3 with a 10 A booster (equipped with a built-in EIS analyzer). The solution resistance (R) in both H-cell and flow cell was evaluated using potentiostatic electrochemical impedance spectroscopy (PEIS). PEIS measurements were conducted before and after the CO(2)R, with a frequency scan range from 1 MHz to 10 Hz. The resulting data were analyzed using EC-Lab software. In the PEIS results, the intersections of the Nyquist plot with the x-axis at the high frequency regions were used to estimate the corresponding solution resistance. All the potential were recorded against Ag/AgCl and then converted to reversible hydrogen electrode (RHE) with iR compensation following the equation below:
For the CO(2)R tests conducted in both H-cell and flow-cell setups, the solution resistance at each applied potential was measured before and after electrolysis to ensure 100% iR compensation. In the membrane electrode assembly (MEA) setup, the full cell voltage was recorded without iR correction.
The Ag/AgCl reference electrode was calibrated using a reversible hydrogen electrode (RHE) in a high-purity H2-saturated 0.5 M H2SO4 electrolyte (pH = 0), with a Pt wire as the working electrode and a graphite rod as the counter electrode. Cyclic voltammetry (CV) was conducted at a scan rate of 1 mV/s, and the thermodynamic potential for the hydrogen evolution reaction (E = 0 V vs. RHE) was determined as the average of the two potentials where the current was zero. The above calibration resulted in a measured RHE value of −0.197 V vs. Ag/AgCl. Hence, −0.197 V was used for converting the applied cathodic potentials to RHE in our experiments.
Different electrolytes were employed in this study. For the H-cell, 0.1/0.5 M KHCO3 was used. For flow-cell tests, the electrolytes included 1 M KOH, 0.5 M K2SO4 (acidic, H2SO4 was used to adjust pH to 2), and 1 M KHCO3 (neutral). For the MEA tests, 0.1 M KHCO3, a 1 M KOH, and 2 M KOH served as neutral and alkaline electrolytes, respectively. Fresh electrolytes were prepared by dissolving the appropriate amounts of KOH, K2SO4, and KHCO3 in 1 L of ultrapure water, stored in a sealed polypropylene bottle. The pH of the solutions was measured using a Mettler-Toledo S400 benchtop pH meter, with the pH values of 0.1 M KHCO3 (CO2 saturated), 0.5 M KHCO3 (CO2 saturated), 1 M KOH, and 2 M KOH being 6.81 ± 0.03, 8.3 ± 0.05, 14.10 ± 0.03, and 14.35 ± 0.05, respectively.
Gaseous products were analyzed and quantified using gas chromatograph (GC, Shimadzu 2014), with the actual gas flow rates, measured by a bubble flowmeter, at the inlet of GC (after outlet of the CO2R reactor) for gaseous product quantification for each single experiments. On the other hand, liquid CO(2)R products were analyzed and quantified by 1H NMR spectrum (Bruker 400 MHZ system) using DMSO and Phenol as the internal standard.
Temperature dependent CO2R experiments
These experiments were performed in a H-Cell purchased from GaossUnion using 0.1 M KHCO3 as the electrolyte. The working electrode (carbon paper, TGP-H-060) in the H-cell was prepared by the drop-coating method. Specially, the catalyst ink was prepared by dispersing 1 mg of catalyst in 1 mL mixture solution (with a water and isopropyl alcohol ratio of 7:3) containing 5 μL of Nafion, followed by thorough ultrasonication. Then, the resulted catalyst ink was coated on the carbon paper (TGP-H-060) with an active area of 1 cm2. The catalyst mass loadings were estimated to be ~1 mg/cm2 by weighing the carbon paper electrodes before and after the catalyst loading after drying. Besides, Pt foil and Ag/AgCl were employed as the reference electrode and counter electrode in the H-cell, respectively. AEM (Sustainion X37-50 RT Grade) was used to separate the cathode and anode chambers. The entire H-cell was placed in a water bath in order to achieve adjustable temperatures. The CO2R assessments were performed for at least 3 times after the water bath temperature was stabilized at the desired temperature for 10 min. During these CO2R tests, the CO2 gas flow rate was regulated by a mass flow controller (MFC, Alicat Scientific) at 25 mL min−1.
CO2/COR measurements in flow cell
All GDEs used in the CO2/COR measurements in flow cell underwent treatment with a PTFE solution to enhance their hydrophobicity. The detailed procedure of this functionalization is as follows: 20 mL of ultrasonically treated 10 wt% PTFE solution was air-brushed evenly onto the gas diffusion layer of commercial GDE carbon paper (YLS-30T, 10 cm2). Subsequently, the spray-coated carbon paper was transferred to a muffle furnace and calcined at 330 °C for 30 min under Ar. After the calcination procedure, the resulted GDE was then used for catalyst loading. It’s important to note that our method can effectively address the variances in hydrophobicity between different batches of carbon paper, even from the same manufacturers, thereby enhancing the stability and repeatability of our catalytic experiments.
The working electrode was prepared through a spray-coating process. Specifically, 10 mg Cu-based catalyst was dispersed in a 2 mL isopropyl alcohol solution, containing 50 μL Nafion, followed by thorough ultrasonication. Subsequently, the catalyst precursor inks were air-brushed onto the micro porous layer of the carbon paper (YLS-30T). The catalyst loadings on each GDE electrodes were controlled to be ~0.6 mg/cm2 by weighing the carbon paper electrodes before and after the catalyst coating with thorough drying.
The CO2/COR performance were assessed using a customized flow electrolytic reactor (Supplementary Fig. 12), with 1 cm2 as the active working electrode area. A reference electrode (Ag/AgCl with double salt bridge) and a counter electrode (Ni foam) were employed in our tests. Anion exchange membrane (AEM), Sustainion X37-50 RT Grade, was used to separate the cathode and anode chambers and the electrolytes. Besides, the electrolyte was circulated by a peristaltic pump at a liquid flow rate of 25 mL min−1. The gas flow rate of CO2 and CO was regulated by an MFC at 25 mL min−1. Regarding the acidic CO2R reaction, 0.5 M K2SO4 (pH = 2) was used as the electrolyte, and a proton exchange membrane (Nafion 117) was used. The other testing conditions were kept the same.
CO2/COR measurements in MEA cell
The working electrode preparation in the MEA tests is nearly identical to what used in the flow cell, with the exception that the catalyst loading was increased to ~1.2 mg cm−2. The MEA (1 cm2 working area, Shanghai leinuo Technology company) setup was illustrated as in Supplementary Fig. 40.
The MEA was fabricated through a hot-pressing procedure involving a cathodic GDE (1 × 1 cm2), an AEM (Sustainion X37-50 RT Grade, 1.5 × 1.5 cm2, thickness 50μm), and an anodic IrOx/Ti mesh (1 × 1 cm2). The hot-pressing was carried out at 70 °C with a pressure of ~ 5 MPa for a duration of 1.5 min. Note that the AEM membrane was activated in 1 M KOH solution for 24 h before use.
In the MEA testing, 0.1 M KHCO3 and 1 M KOH were used as the electrolytes for CO2R. As for COR, 2 M KOH were used the electrolyte. The anolyte temperature was kept as 80 °C to facilitate the oxygen evolution reaction (OER). The electrolyte flow rate was regulated by a peristaltic pump at a rate of 50 mL min−1. The gas flow rate of CO2/CO was controlled by an MFC at 25 mL min−1.
Faradic efficiency
The Faradic Efficiency (FE) of each product in CO2R or COR were estimated using the equation below:
where n is the number of electrons needed for a specific product, F is the Faradaic constant (96,485 C mol−1). The amount of products (mol) were quantified by GC and NMR accordingly. C denotes for the total charge passing through during the catalysis.
Cathodic energy efficiency of C2+ products (EE1/2)
For a specific C2+ product in CO(2)R, such as ethylene, ethanol, acetate, and n-propanol, the half-cell energy efficiency of this product was estimated by the equation below:
Where the \({E}_{{CO}2/{product}}^{0}\) and \({E}_{{OER}}^{0}\) are the thermodynatic potentials for CO2R to a specific product (cathodic reaction) and OER (anodic reaction), respectivley. FE is the faradic efficiency for the product measured at a given potentials. The thermodynatic potential for OER is 1.23 V vs. RHE. The themrodynamic potentials for different CO2R products are listed in Supplementary Table 7. And the EE1/2 of C2+ products is the sum of all individal proudcts.
Full cell energy efficiency of C2+ products
The full-cell energy efficiency for CO(2)R products was calculated by follow equation:
Where \({E}_{A}^{0}\) is the thermodynatic potential of CO(2) to the specific product10. \({E}_{{Ethylene}}^{0}\) = 0.17 VRHE; \({E}_{{Ethanol}}^{0}\) = 0.178 VRHE; \({E}_{{acetate}}^{0}\) = 0.454 VRHE; \({E}_{n-{PrOH}}^{0}\) = 0.2 VRHE. FE is the faradic efficiency for A at different potentials. \({E}_{{cell\; voltage}}^{{Applied}}\) is the measured cell voltage in the MEA system without any iR compensations.
Single-pass carbon efficiency (SPCE)
The SPCE for CO2R was estimated based on the following equation:
Where I is the partial current density, FEA is the FE of A, \({x}_{i}\) is mole ratio of CO2 to products (\({x}_{i}\) = 1 for C1 products, \({x}_{i}\) = 2 for C2 products), n is the number of electrons needed for a specific produce, F is the Faradaic constant.
DFT calculations
All the calculations were performed with VASP at the spin-polarized GGA level with the Perdew−Burke−Ernzerhof exchange−correlation functional, and the projector-augmented-wave pseudopotential74,75,76. An energy cutoff of 400 eV was applied for the plan-wave basis set75. Cu(100) surface was selected because it has been proposed to be responsible for the C-C coupling77. The surface was described by a three-layer slab model with a vacuum layer of 14 Å. A 4 × 4 supercell was used with the Brillouin zone sampled by a 3 × 3 grid of Monkhorst-Pack k-points78. For the surface with 1/6 monolayer (ML) of Ga, a 3 × 4 supercell was used with a 4 × 3 grid of Monkhorst-Pack k-points. The dispersive interaction was treated with the DFT + D3 scheme79. Solvation effects were included with the implicit solvation model within VASPsol80. With the atoms in the bottom layer fixed to the bulk positions, the atoms in the up-two layers were allowed to relax until the maximum force on the atoms was smaller than 0.02 eV/Å. To simulate the CO-CO coupling process, two water molecules were adsorbed on the surface to stabilize the OCCO intermediates. Transition states were searched with the nudged elastic band method with climbing images81. The free energy profiles were calculated with the computational hydrogen electrode model82. Free energy corrections (including the zero-point energy, enthalpy, and entropy contributions) were included in the harmonic approximation59. The gas-phase molecules were treated as ideal gases. Energy corrections of 0.13 eV and –0.29 eV was applied to the CO2 and CO molecules such that the calculated gas-phase thermochemical reaction enthalpies are consistent with the experimental values83.
Data availability
Source data of this paper are provided in this paper and are also available from the corresponding authors upon request. Source data are provided with this paper.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Li, J. et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat. Catal. 2, 1124–1131 (2019).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Chen, L. et al. Additive-assisted electrodeposition of Cu on gas diffusion electrodes enables selective CO2 reduction to multicarbon products. ACS Catal. 13, 11934–11944 (2023).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Wang, P. et al. Boosting electrocatalytic CO2–to–ethanol production via asymmetric C–C coupling. Nat. Commun. 13, 3754 (2022).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Wang, X. et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Nat. Energy 7, 170–176 (2022).
Niu, W. et al. Pb-rich Cu grain boundary sites for selective CO-to-n-propanol electroconversion. Nat. Commun. 14, 4882 (2023).
Ozden, A. et al. Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption. Nat. Energy 8, 179–190 (2023).
Zhang, J. et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. Nat. Commun. 14, 1298 (2023).
Salvatore, D. & Berlinguette, C. P. Voltage matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 215–220 (2020).
Fan, L. et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 6, eaay3111 (2020).
Wang, Y., Han, P., Lv, X., Zhang, L. & Zheng, G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule 2, 2551–2582 (2018).
Lee, S. Y. et al. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2. Reduct. J. Am. Chem. Soc. 140, 8681–8689 (2018).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Huang, J., Mensi, M., Oveisi, E., Mantella, V. & Buonsanti, R. Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag–Cu nanodimers. J. Am. Chem. Soc. 141, 2490–2499 (2019).
Feng, J. et al. Improving CO2-to-C2+ product electroreduction efficiency via atomic lanthanide dopant-induced tensile-strained CuOx catalysts. J. Am. Chem. Soc. 145, 9857–9866 (2023).
Zheng, M. et al. Electrocatalytic CO2-to-C2+ with ampere-level current on heteroatom-engineered copper via tuning *CO intermediate coverage. J. Am. Chem. Soc. 144, 14936–14944 (2022).
Deng, B., Huang, M., Zhao, X., Mou, S. & Dong, F. Interfacial electrolyte effects on electrocatalytic CO2 reduction. ACS Catal. 12, 331–362 (2022).
Fang, M. et al. Hydrophobic, Ultrastable Cuδ+ for robust CO2 electroreduction to C2 products at ampere-current levels. J. Am. Chem. Soc. 145, 11323–11332 (2023).
Ge, L. et al. Electrochemical CO2 reduction in membrane-electrode assemblies. Chem 8, 663–692 (2022).
Zhang, Z. et al. Membrane electrode assembly for electrocatalytic CO2 reduction: principle and application. Angew. Chem., Int. Ed. Engl. 62, e202302789 (2023).
Weng, L.-C., Bell, A. T. & Weber, A. Z. Towards membrane-electrode assembly systems for CO2 reduction: a modeling study. Energy Environ. Sci. 12, 1950–1968 (2019).
Lees, E. W. et al. Exploring CO2 reduction and crossover in membrane electrode assemblies. Nat. Chem. Eng. 1, 340–353 (2024).
Feng, J. et al. Modulating adsorbed hydrogen drives electrochemical CO2-to-C2 products. Nat. Commun. 14, 4615 (2023).
Liu, Z. et al. Interfacial water tuning by intermolecular spacing for stable CO2 electroreduction to C2+ products. Angew. Chem., Int. Ed. Engl. n/a, e202309319 (2023).
Gileadi, E. Physical Electrochemistry. Fundamentals, Techniques, and Applications. (Wiley-VCH, 2011). ISBN: 978-3-527-31970-1.
Gileadi, E. Physical Electrochemistry. Fundamentals, Techniques and Applications. (Gulf Professional Publishing, 2021). ISBN:9780128243992.
Yang, H. et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities. Nat. Commun. 11, 593 (2020).
Hua, W. et al. A hierarchical Single-Atom Ni-N3-C catalyst for electrochemical CO2 reduction to CO with Near-Unity faradaic efficiency in a broad potential range. Chem. Eng. J. 446, 137296 (2022).
Zhang, X. et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat. Energy 5, 684–692 (2020).
Zheng, T. et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16, 1386–1393 (2021).
Zhao, Y. et al. Spontaneously Sn-doped Bi/BiOx Core–Shell nanowires toward high-performance CO2 electroreduction to liquid fuel. Nano Lett. 21, 6907–6913 (2021).
Jia, B. et al. Indium cyanamide for industrial-grade CO2 electroreduction to formic acid. J. Am. Chem. Soc. 145, 14101–14111 (2023).
Joensen, B. Ó. et al. Unveiling transport mechanisms of cesium and water in operando Zero-Gap CO2 electrolyzers. Joule 8, 1–18 (2024).
Yang, K., Kas, R., Smith, W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 6, 33–40 (2021).
Kim, B. et al. Trace-level cobalt dopants enhance CO2 electroreduction and ethylene formation on copper. ACS Energy Lett. 8, 3356–3364 (2023).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Wei, Z. et al. Enhancing selective electrochemical CO2 reduction by in situ constructing tensile-strained Cu catalysts. ACS Catal. 13, 4711–4718 (2023).
Hansen, H. A., Shi, C., Lausche, A. C., Peterson, A. A. & Nørskov, J. K. Bifunctional alloys for the electroreduction of CO2 and CO. Phys. Chem. Chem. Phys. 18, 9194–9201 (2016).
Guan, A. et al. Steric effect induces CO electroreduction to CH4 on Cu–Au alloys. J. Mater. Chem. A 9, 21779–21784 (2021).
Zhao, J. et al. Modulation of *CHxO adsorption to facilitate electrocatalytic reduction of CO2 to CH4 over Cu-Based Catalysts. J. Am. Chem. Soc. 145, 6622–6627 (2023).
Castilla-Amorós, L., Chien, T.-C. C., Pankhurst, J. R. & Buonsanti, R. Modulating the reactivity of liquid Ga nanoparticle inks by modifying their surface chemistry. J. Am. Chem. Soc. 144, 1993–2001 (2022).
Liu, H. et al. Solid–liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction. Nat. Catal. 4, 202–211 (2021).
Zhan, P. et al. Synthesis of AuX (X = Ni, Ga, Mo, Zn, and Cr) alloy aerogels as high-performance electrocatalytic CO2 reduction reaction catalysts. Langmuir 39, 8306–8313 (2023).
Tran, K. & Ulissi, Z. W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 1, 696–703 (2018).
Okatenko, V. et al. The native oxide skin of liquid metal ga nanoparticles prevents their rapid coalescence during electrocatalysis. J. Am. Chem. Soc. 144, 10053–10063 (2022).
Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 145, 5370–5383 (2023).
Li, P. et al. p–d Orbital hybridization induced by p-block metal-doped Cu promotes the formation of C2+ products in ampere-level CO2 electroreduction. J. Am. Chem. Soc. 145, 4675–4682 (2023).
Yan, S. et al. High-power CO2-to-C2 electroreduction on Ga-spaced, square-like Cu sites. J. Am. Chem. Soc. 145, 26374–26382 (2023).
Ummireddi, A. K., Li, Z. & Wu, J. Copper defects for CO2 electrocatalysis toward a specific multicarbon product. Trends Chem. 5, 170–173 (2023).
Cheng, D. et al. The nature of active sites for carbon dioxide electroreduction over oxide-derived copper catalysts. Nat. Commun. 12, 395 (2021).
Chen, Z. et al. Grain-boundary-rich copper for efficient solar-driven electrochemical CO2 reduction to ethylene and ethanol. J. Am. Chem. Soc. 142, 6878–6883 (2020).
Zong, Y., Chakthranont, P. & Suntivich, J. Temperature effect of CO2 reduction electrocatalysis on copper: potential dependency of activation energy. J. Electrochem. En. Conv. Stor 17, 041007 (2020).
Vos, R. E. et al. How temperature affects the selectivity of the electrochemical CO2 reduction on copper. ACS Catal. 13, 8080–8091 (2023).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Wu, H. et al. Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization. Nat. Energy 9, 422–433 (2024).
Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B. The role of in situ generated morphological motifs and Cu(I) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 5, 317–325 (2020).
Li, J. et al. Selective CO2 electrolysis to CO using isolated antimony alloyed copper. Nat. Commun. 14, 340 (2023).
Bratsch, S. G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1–21 (1989).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C. 121, 12337–12344 (2017).
Yang, X. et al. Mechanistic insights into C-C coupling in electrochemical CO reduction using gold superlattices. Nat. Commun. 15, 720 (2024).
Hou, J. et al. Influence of electric double layer rigidity on CO adsorption and electroreduction rate. Nat. Commun. 15, 1926 (2024).
Ma, W. et al. Copper lattice tension boosts full-cell CO electrolysis to multi-carbon olefins and oxygenates. Chem 9, 2161–2177 (2023).
Cheng, T., Xiao, H. & Goddard, W. A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 114, 1795–1800 (2017).
Eilert, A. et al. Subsurface oxygen in oxide-derived copper electrocatalysts for carbon dioxide reduction. J. Phys. Chem. Lett. 8, 285–290 (2017).
Kim, J. Y. T., Sellers, C., Hao, S., Senftle, T. P. & Wang, H. Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions. Nat. Catal. 6, 1115–1124 (2023).
Zhang, T. et al. The conventional gas diffusion electrode may not be resistant to flooding during CO2/CO Reduction. J. Electrochem. Soc. 169, 104506 (2022).
Popović, S. et al. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 Reduction. Angew. Chem., Int. Ed. Engl. 59, 14736–14746 (2020).
Vavra, J., Shen, T.-H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem., Int. Ed. Engl. 60, 1347–1354 (2021).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Schouten, K. J. P., Qin, Z., Pérez Gallent, E. & Koper, M. T. M. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J. Am. Chem. Soc. 134, 9864–9867 (2012).
Monkhorst, H. J. & Pack, J. D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 13, 5188–5192 (1976).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 32, 1456–1465 (2011).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Han, N. et al. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 9, 1320 (2018).
Acknowledgements
We acknowledge the National University of Singapore and Ministry of Education for their financial support, through the grants A-0009176-02-00 and A-0009176-03-00, as well as A*STAR (Agency for Science, Technology and Research) under its LCERFI program (award no. U2102d2002). We would also like to acknowledge the support of the National Research Foundation (NRF) Singapore, under the NRF Fellowship (NRF-NRFF13-2021-0007) and CRP (NRF-CRP27-2021-0004), as well as the support from the Center for Hydrogen Innovations at the NUS (CHI-P2022-06; A-8000138-03-00). Y.J. also would like to acknowledge the Natural Science Foundation of Guangdong Province (2023A1515012238), and Guangzhou Municipal Science and Technology Bureau (202201020145).
Author information
Authors and Affiliations
Contributions
L.W. and L.C. conceived and designed this project. Y.J. conducted the computational calculation and relative data analysis. L.C., J.C., W.F., and D.W. synthesized the catalysts and conducted the electrochemical experiments. L.C. and Y.X. carried out the ex-situ physical characterizations. L.C. and J.C. conducted the operando XAS experiments under the supervision of S.X. Everyone contributed to data analysis, writing, and editing of the manuscript. L.W. supervised the entire project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xuan Yang, Siddhartha Subramanian, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, L., Chen, J., Fu, W. et al. Energy-efficient CO(2) conversion to multicarbon products at high rates on CuGa bimetallic catalyst. Nat Commun 15, 7053 (2024). https://doi.org/10.1038/s41467-024-51466-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51466-8
This article is cited by
-
Thousand-hour salt precipitation-free CO2-to-ethylene electrosynthesis at high current densities
Nature Communications (2025)
-
A chelate to break diffusion limits on Helmholtz plane for CO2 electroreduction to ethanol
Nature Communications (2025)
-
Linking electrocatalytic turnover to elementary step rates in hydrocarbon fuel oxidation
Nature Communications (2025)
-
Scalable metal–organic framework-based electrodes for efficient alkaline water electrolysis
Nature Chemical Engineering (2025)
-
Optimizing water dissociation through doping fluorine into La2CuO4 to enhance multicarbon generation in CO2 electroreduction
Science China Materials (2025)